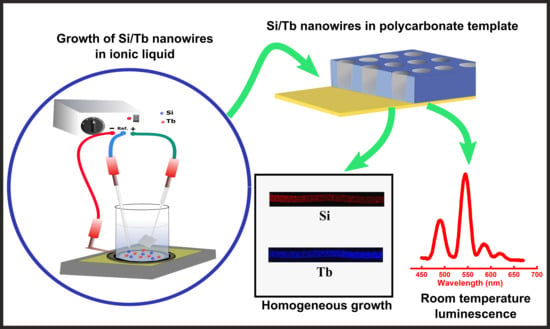
Growth of Homogeneous Luminescent Silicon–Terbium Nanowires by One-Step Electrodeposition in Ionic Liquids
Abstract
:Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, Y.; Lieber, C.M. Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.-Q.; Lee, S.-T. Silicon Nanowires for Photovoltaic Solar Energy Conversion. Adv. Mater. 2011, 23, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-Y.; Anthony, R.; Kortshagen, U.R.; Holmes, R.J. High-Efficiency Silicon Nanocrystal Light-Emitting Devices. Nano Lett. 2011, 11, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Bowers, J.E. Recent progress in lasers on silicon. Nat. Photon. 2010, 4, 511–517. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Zheng, G.; Gao, X.P.A.; Lieber, C.M. Frequency Domain Detection of Biomolecules Using Silicon Nanowire Biosensors. Nano Lett. 2010, 10, 3179–3183. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, A.J. Erbium in silicon. Semicond. Sci. Technol. 2005, 20, R65–R84. [Google Scholar] [CrossRef]
- Bahuleyan, B.K.; Toussaint, K.; Rinnert, H.; Vallon, R.; Molinari, M.; Chuburu, F.; Cadiou, C. Silicon Wafer Functionalization with a Luminescent Tb (III) Coordination Complex: Synthesis, Characterization, and Application to the Optical Detection of NO in the Gas Phase. Molecules 2019, 24, 1914. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, Y.; Huang, L.; Lu, P.; Sun, Q.; Wang, Y.; Tang, J.; Belfiore, L.A.; Kipper, M.J. Polyvinylpyrrolidone Nanofibers Encapsulating an Anhydrous Preparation of Fluorescent SiO2–Tb3+ Nanoparticles. Nanomaterials 2019, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Mula, G.; Setzu, S.; Manunza, G.; Ruffilli, R.; Falqui, A. Optical, electrochemical, and structural properties of Er-doped porous silicon. J. Phys. Chem. C 2012, 116, 11256–11260. [Google Scholar] [CrossRef]
- Irrera, A.; Faro, M.J.L.; D’Andrea, C.; Leonardi, A.A.; Artoni, P.; Fazio, B.; Picca, R.A.; Cioffi, N.; Trusso, S.; Franzò, G. Light-emitting silicon nanowires obtained by metal-assisted chemical etching. Semicond. Sci. Technol. 2017, 32, 043004. [Google Scholar] [CrossRef]
- Huang, C.; Hsin, C.; Huang, K.; Lee, C.; Yeh, P.; Chen, U.; Chen, L. Er-doped silicon nanowires with 1.54 μm light-emitting and enhanced electrical and field emission properties. Appl. Phys. Lett. 2007, 91, 093133. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-J.; Shin, J.H.; Suh, K.; Seong, H.-K.; Han, H.-C.; Lee, J.-C. Self-Organized Growth of Si/Silica/Er2Si2O7 Core− Shell Nanowire Heterostructures and their Luminescence. Nano Lett. 2005, 5, 2432–2437. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Zhang, J.; Gao, J.; Ding, X.; Huang, Z.; Tang, C.; Hu, L.; Chen, D. Characterization and photoluminescence properties of Tb-doped SiO2 nanowires as a novel green-emitting phosphor. Chem. Mater. 2007, 19, 2585–2588. [Google Scholar] [CrossRef]
- Singha, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. Visible detection of explosive nitroaromatics facilitated by a large stokes shift of luminescence using europium and terbium doped yttrium based MOFs. RSC Adv. 2015, 5, 102076–102084. [Google Scholar] [CrossRef]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef]
- Dumont, L.; Cardin, J.; Benzo, P.; Carrada, M.; Labbe, C.; Richard, A.L.; Ingram, D.C.; Jadwisienczak, W.M.; Gourbilleau, F. SiNx: Tb3+–Yb3+, an efficient down-conversion layer compatible with a silicon solar cell process. Sol. Energy Mater. Sol. Cells 2016, 145, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Berning, G.; Swart, H. Multilayer formation during annealing of thin Tb layers on SiO2 substrates. Surf. Interface Anal. 1998, 26, 420–424. [Google Scholar] [CrossRef]
- Kaleli, B.; Kulakci, M.; Turan, R. Mechanisms of light emission from terbium ions (Tb3+) embedded in a Si rich silicon oxide matrix. Opt. Mater. 2012, 34, 1935–1939. [Google Scholar] [CrossRef]
- Gao, W.; Li, T.-k.; Ono, Y.; Hsu, S.-T. Photoluminescence and electroluminescence studies on Tb-doped silicon rich oxide materials and devices. J. Rare Earths 2006, 24, 673–678. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Shin, J.H. Enhancement of the green, visible Tb3+ luminescence from Tb-doped silicon-rich silicon oxide by C co-doping. Appl. Phys. Lett. 2004, 84, 4379–4381. [Google Scholar] [CrossRef] [Green Version]
- Gelloz, B.; Mentek, R.; Koshida, N. Optical properties of phosphorescent nano-silicon electrochemically doped with terbium. Phys. Status Solidi c 2012, 9, 2318–2321. [Google Scholar] [CrossRef]
- Elhouichet, H.; Oueslati, M. Rare earth ions in porous silicon: Optical properties. Phys. Status Solidi A 2007, 204, 1497–1501. [Google Scholar] [CrossRef]
- den Hertog, M.I.; Rouviere, J.-L.; Dhalluin, F.; Desré, P.J.; Gentile, P.; Ferret, P.; Oehler, F.; Baron, T. Control of Gold Surface Diffusion on Si Nanowires. Nano Lett. 2008, 8, 1544–1550. [Google Scholar] [CrossRef]
- Labbe, C.; An, Y.-T.; Zatryb, G.; Portier, X.; Podhorodecki, A.; Marie, P.; Frilay, C.; Cardin, J.; Gourbilleau, F. Structural and emission properties of Tb3+-doped nitrogen-rich silicon oxynitride films. Nanotechnology 2017, 28, 115710. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, D.; Mallet, J.; Thomas, S.; Nemaga, A.W.; Michel, J.; Guery, C.; Molinari, M.; Morcrette, M. Electrochemical synthesis of 1D core-shell Si/TiO2 nanotubes for lithium ion batteries. J. Power Sources 2017, 361, 243–248. [Google Scholar] [CrossRef]
- Mallet, J.; Molinari, M.; Martineau, F.; Delavoie, F.; Fricoteaux, P.; Troyon, M. Growth of Silicon Nanowires of Controlled Diameters by Electrodeposition in Ionic Liquid at Room Temperature. Nano Lett. 2008, 8, 3468–3474. [Google Scholar] [CrossRef]
- Mallet, J.; Martineau, F.; Namur, K.; Molinari, M. Electrodeposition of silicon nanotubes at room temperature using ionic liquid. Phys. Chem. Chem. Phys. 2013, 15, 16446–16449. [Google Scholar] [CrossRef]
- Al-Salman, R.; Mallet, J.; Molinari, M.; Fricoteaux, P.; Martineau, F.; Troyon, M.; El Abedin, S.Z.; Endres, F. Template assisted electrodeposition of germanium and silicon nanowires in an ionic liquid. Phys. Chem. Chem. Phys. 2008, 10, 6233–6237. [Google Scholar] [CrossRef]
- Fournier, C.; Favier, F. Zn, Ti and Si nanowires by electrodeposition in ionic liquid. Electrochem. Commun. 2011, 13, 1252–1255. [Google Scholar] [CrossRef]
- Thomas, S.; Mallet, J.; Rinnert, H.; Molinari, M. Single step electrodeposition process using ionic liquid to grow highly luminescent silicon/rare earth (Er, Tb) thin films with tunable composition. RSC Adv. 2018, 8, 3789–3797. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Mallet, J.; Martineau, F.; Rinnert, H.; Molinari, M. Strong Room-Temperature Visible Photoluminescence of Amorphous Si Nanowires Prepared by Electrodeposition in Ionic Liquids. ACS Photonics 2018, 5, 2652–2660. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Berning, G.L.P.; Swart, H.C.; de Witt, B. An Auger electron spectroscopy study of the sputtering and room-temperature oxidation of terbium and terbium silicide. Appl. Surf. Sci. 1993, 64, 1–7. [Google Scholar] [CrossRef]
- Podhorodecki, A.; Zatryb, G.; Misiewicz, J.; Wojcik, J.; Wilson, P.R.J.; Mascher, P. Green light emission from terbium doped silicon rich silicon oxide films obtained by plasma enhanced chemical vapor deposition. Nanotechnology 2012, 23, 475707. [Google Scholar] [CrossRef]
- Kam, C.; Buddhudu, S. Emission properties of GdOBr: Ce3+ and Tb3+ phosphors. Mater. Lett. 2002, 54, 337–342. [Google Scholar] [CrossRef]
- Roschuk, T.; Wilson, P.R.J.; Li, J.; Zalloum, O.H.Y.; Wojcik, J.; Mascher, P. Structure and luminescence of rare earth-doped silicon oxides studied through their X-ray absorption near edge structure and X-ray excited optical luminescence. Phys. Status Solidi B 2010, 247, 248–253. [Google Scholar] [CrossRef]
- Amekura, H.; Eckau, A.; Carius, R.; Buchal, C. Room-temperature photoluminescence from Tb ions implanted in SiO2 on Si. J. Appl. Phys. 1998, 84, 3867–3871. [Google Scholar] [CrossRef]
- Yacobi, B.G.; Holt, D.B. Cathodoluminescence. In Cathodoluminescence Microscopy of Inorganic Solids; Springer: New York, NY, USA, 1990; pp. 55–88. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Mallet, J.; Bahuleyan, B.K.; Molinari, M. Growth of Homogeneous Luminescent Silicon–Terbium Nanowires by One-Step Electrodeposition in Ionic Liquids. Nanomaterials 2020, 10, 2390. https://doi.org/10.3390/nano10122390
Thomas S, Mallet J, Bahuleyan BK, Molinari M. Growth of Homogeneous Luminescent Silicon–Terbium Nanowires by One-Step Electrodeposition in Ionic Liquids. Nanomaterials. 2020; 10(12):2390. https://doi.org/10.3390/nano10122390
Chicago/Turabian StyleThomas, Shibin, Jeremy Mallet, Bijal K. Bahuleyan, and Michael Molinari. 2020. "Growth of Homogeneous Luminescent Silicon–Terbium Nanowires by One-Step Electrodeposition in Ionic Liquids" Nanomaterials 10, no. 12: 2390. https://doi.org/10.3390/nano10122390
APA StyleThomas, S., Mallet, J., Bahuleyan, B. K., & Molinari, M. (2020). Growth of Homogeneous Luminescent Silicon–Terbium Nanowires by One-Step Electrodeposition in Ionic Liquids. Nanomaterials, 10(12), 2390. https://doi.org/10.3390/nano10122390