Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies?
Abstract
:1. Introduction
1.1. Progress in InChI Representation of Small Molecules, Polymers, Mixtures and Reactions
1.2. A Proposal for a Hierarchical Representation of Chemical and Structural Complexity of NMs
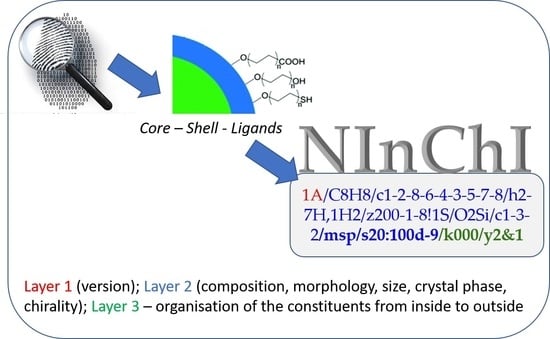
1.3. What We Intended the Case Studies to Teach Us
- (i)
- distinguishes between NMs and groups similar NMs;
- (ii)
- enables extraction of the main characteristics of the NM directly from the representation. This is a machine-readable form usable by databases and literature-mining tools that can guide users to information on specific NMs that discriminates between bulk forms of materials or other NMs with other sizes, shapes, or surface modifications;
- (iii)
- allows users to merge relevant information from different sources, including data from other NMs with varying degrees of similarity to the material under study; and
- (iv)
- can be unequivocally decoded into structural information used to generate data-driven and physics-based computational models.
2. Materials and Methods
2.1. Experimental Case Studies
2.1.1. Case Study 1. Functionalized Gold NMs
2.1.2. Case Study 2. Graphene-Family NMs
2.1.3. Case Study 3. Complex Engineered (Doped and Multi-Metallics) NMs
2.2. Application Use Case Studies
2.2.1. Case Study 4. Encoding for Data FAIRness
2.2.2. Case Study 5. Nanoinformatics Applications
2.2.3. Case Study 6. NM Regulation
2.3. Identifying Essential NInChI Features by an Iterative Prioritization Process
3. Results and Discussion
3.1. Case Study 1. Functionalized Gold NMs
3.1.1. Objective
3.1.2. Specific Features of Functionalized Au NMs
- Chemical composition. The simplest NMs were spheroid Au cores with an organic surface ligand for stabilization. The size and shape of Au NPs were controlled by changing the synthesis conditions [72]. Chemical information is processed from the centre out, starting with basic information about core composition, size, and shape, before progressing to surface characteristics and functionalities, and even extending to interactions with surrounding molecules.
- ➢
- Thus, minimum information is the core composition and a representation of the stabilizing ligand.
- Size, shape and morphology. NMs are in general referred to by their core composition, with an associated physicochemical property and/or morphology, e.g., Au quantum dots, citrate-functionalized Au NMs, core-shell silver-gold NMs, etc. Their biological effects and physicochemical properties depend on the NM dimensions and shapes, e.g., spheres, rods, stars, cages, core-shell particles, etc. At the atomic level, a multitude of Au NM shapes can be differentiated [73]. Au nanospheres or colloids can be synthesized in an aqueous HAuCl4 solution using different reducing agents, e.g., citrate, which produces nearly monodisperse nanospheres [74]. The size of the nanospheres can be precisely controlled by varying the citrate/Au ratio, i.e., smaller amounts of citrate will yield larger nanospheres, and size variants differing by only few nm have been successfully synthesized [75,76]. Alternatively, Au nanorods are synthesized using templates, e.g., the electrochemical deposition of Au within the pores of nanoporous polycarbonate, or, alumina template membranes [77,78]. The pore diameter of the template membrane determines the diameter of the Au nanorod. The length of the nanorod can be controlled by the amount of Au deposited within the pores of the template [74]. Au nanostars have a thin, branch-like structure exhibiting plasmonic properties [79] and enhanced near-infrared light-absorbing capabilities, with reduced toxicity [80]. Octahedral solid core Au nanohexapods have been fabricated by reducing HAuCl4 with DMF in an aqueous solution containing Au octahedral seeds [81,82]. Case study 1 suggested that the core size, shape and nanotopography of the Au NMs (intrinsic properties) should be determined by direct imaging techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) [83] (see Table S1) [84,85,86,87,88]. However, there are alternative ways to report size and shape and their distributions. Since Au NMs are not completely monodisperse, it is important to determine the particle size distribution to determine how agglomerate size affects toxicity [89], or to assess the quality of synthesized Au NPs [90]. Dynamic light scattering (DLS) is the most common sizing technique but has limitations, e.g., high polydispersity can distort the results [91]. Thus, consensus approaches to describing size distributions may be needed, e.g., DLS, SEM/TEM, field flow fractionation coupled to online sizing detectors, centrifugal techniques, nanoparticle tracking analysis and tunable resistive pulse sensing [50]. However, commonly the mean diameter is reported [84,92,93].
- ➢
- The structural representation of a NM must include size, size distribution, shape, and morphology distinguish one NM from another.
- Dimension and thickness of coating or shell. This property relates to chemical composition and morphology and may be difficult to determine. Examples include NMs where silica or polymer beads are coated with Au of variable thickness, creating Au nanoshells [94,95]. The diameter of the nanoshell is determined by the diameter of the underlying core, and the shell thickness is controlled by the amount of Au deposited on the surface of the core [74]. By varying the composition and dimensions of the chemical layers, nanoshells can be fabricated with surface plasmon resonance (SPR) peaks ranging from the visible to the near-infrared region, i.e., 700–900 nm [96]. Similarly, nanocages are hollow, porous Au NMs ranging in size from 10 to >150 nm. Silver nanostructures can be used as a sacrificial template and transformed into Au nanostructures with hollow interiors via galvanic replacement, e.g., a reaction between truncated silver nanocubes and aqueous HAuCl4 [96,97]. Au nanocages have been created with controllable pores on the surface [98]. The dimension and wall thickness of the nanocage is controlled by adjusting the molar ratio of silver to HAuCl4 [74]. Gold nanocages also be heated by light (photothermal effect) [82].
- ➢
- These examples show that a shell formalization is needed that captures the dimensions and features of each shell in a sequential manner based on distance from the core, i.e., core-shell1-shell2 etc.
- Au NM surface characteristics and functionalities—Experimental determination of all relevant physicochemical NM surface characteristics such as roughness, charge density, oxidation, etc. is often infeasible so capturing these properties in a NInChI appears beyond the scope. This is less a limitation for intentionally synthesized conjugates. For example, PEGylation involves coating NMs by grafting, entrapping, adsorbing, or covalently binding to the NM surface to enhance its stabilization [99,100]. Covering Au NMs with polyethylene glycol (PEG) or its derivatives modifies binding of plasma proteins, interaction with opsonins, and clearance by the reticuloendothelial system [72]. Similarly, nanoflares are Au conjugates functionalized with oligonucleotide sequences complementary to a specific nucleic acid target (messenger RNA) hybridized to short sequences that fluoresce when bound to a target [101].
- ➢
- These examples indicate the need to describe organic coatings or biomolecules. However, quantitative and qualitative description of the coating entity (density, thickness, purity, orientation, bonding) may be beyond the scope of the NInChI, at least for now.
- Au NM interactions with surrounding molecules—The surface characteristics of Au NMs determine their life span and fate within the body and their toxicity [99,100,102,103]. As noted above, Au NP toxicity is affected by the type of particle coating [103], with polymer coatings increasing the stability and prolonging the NM circulation in the blood by reducing binding of opsonizing proteins [72]. Surface characteristics can influence the electrostatic and hydrophobic interactions between particles (i.e., agglomeration) and clearance by opsonization toxicity [99,100,102,103]. A protein corona can form on particles in vivo [104] that influences biodistribution, biokinetics and toxicity. An overview of toxicity studies of different Au NMs and their functionalizations is given in Table S3 which identifies the need for detailed structural representation of the ligands and surface functionalization.
- ➢
- Although relevant to the biological behavior of the NMs, protein corona and interactions with the environment are extrinsic properties or transformations that are beyond the scope of the proposed NInChI. They may, however, be suitable for a future extension by analogy with the RInChI.
3.1.3. Conclusions on Relevant Features of a NInChI
3.2. Case Study 2. Graphene-Family NMs
3.2.1. Objective
3.2.2. Specific Features of Graphene-Family NMs
- ➢
- Graphene introduces new structural features necessary to differentiate between members. These include edge-structures, impurities, and defects (Tiers 2 and 3 in Figure 1). MInChI may allow inclusion of impurities in a NInChI, while edges and defects could be defined as new categories. RInChI may facilitate grouping of synthesis-route specific properties if they are reproducible.
- ➢
- These examples further elaborate formalization of hollow NMs representations.
- ➢
- As surface functionalization and doping by heteroatoms can alter the properties of CNTs, (see also case study 3) a mechanism to capture the bonding modality in a NInChI would be useful, (Tier 4 in Figure 1).
3.2.3. Conclusions on Relevant Features of a NInChI
- Graphene. The proposed NInChI should indicate: the size of the graphene layer(s); the number of layers (single, bi-, tri-, n- layers); the topology of the structure if applicable (zig-zag, armchair); surface/edge functionalization and bonding mode; impurity information; and heteroatom doping. Although defects affect the properties of graphene, incorporation of information about them may be too difficult, especially for the non-intentional defects.
- Fullerenes. InChI notations for Cn (C60–C90) are already well established. However, to represent fullerenes as part of graphene-family materials and properly identify derivatives and surface modifications, NInChI could add as additional extensions that describe structural changes (i.e., the identity of surface functionalizations).
- CNTs. Graphene may be considered the parent material for CNTs, which are essentially folded (rolled) graphene sheets. Consequently, the NInChI will share common attributes with graphene sheet(s), such as: surface and edge functionalization; heteroatomic doping; and information on impurities. Additionally, information on the number of walls within the CNT should be provided (e.g., SWCNT, DWCBT or MWCNT). CNTs also require more extensive description of their surface and morphology. Therefore, the NInChI should be extended to include information on: the nanotube chirality defined by (n,m) notation (ideally each layer in the MWCNT should have its chirality defined); outer and inner diameters; length; surface charge; and specific surface area. CNTs exhibit additional, higher-level morphological properties to be included (to some extent) in the NInChI, e.g., the end-capping of nanotubes and their shape. End-capping could be partially covered by the edge functionalization parameter described above. However, this refers to the substances or heteroatoms bonded to the edges of CNTs, and should be distinguished from the outer carbon-capping of the tube structure. Due to the complexity of the capping parameter (studies show that the curvature of the cap can alter the properties of the CNT), only basic information on whether the nanotube is closed or open could be accommodated. Finally, information on the shape of the tubular structure should be included in the NInChI notation e.g., straight, branched, helical, waved, and more [119]. This could be accommodated by shape classes that group similar types of tubes.
3.3. Case Study 3. Complex Engineered (Doped and Multi-Metallics) NMs
3.3.1. Objective
3.3.2. Specific Features of Complex Engineered NMs
- ➢
- These structures highlight a need to capture information on crystal structures, which may be mixtures of phases, and on amounts of dopants and distribution in the NMs. These features map to Tier 1 in Figure 1.
3.3.3. Conclusions on Relevant Features of a NInChI
3.4. Case Study 4. Encoding for Data FAIRness
3.4.1. Objective
3.4.2. Specific Use Cases—Nano-Related Data Management, Analysis and “FAIRification”
- ➢
- NInChI will enhance FAIRness of NMs datasets by providing a higher level of indexing based on information in the different layers. Specific NMs can be found based on size, shape, surface coating etc. as encoded in the NInChI. Similarly, scientific interoperability of datasets will be enhanced by exclusion of non-relevant datasets.
- ➢
- If a NInChI is used to establish similarity of NMs (across batches, following storage or ageing, etc.) its representation should include additional methods for determining the relevant properties to ensure direct comparability/interoperability. We therefore argue for a NInChI encoding sufficient information to quickly gauge similarity that is adequate for most applications that integrates measurements from multiple batches and samples of NMs.
- ➢
- NInChIs can support the integration of computational NMs and their associated simulation datasets into nanosafety databases, with transparency around the origin of NMs datasets (experimental versus computational), through inclusion of notation to indicate in silico NMs.
3.4.3. Conclusions on Relevant Features of a NInChI
3.5. Case Study 5. Nanoinformatics Applications
3.5.1. Objective
3.5.2. Specific Use Cases—Calculating Nanodescriptors and Enabling Read-Across
- ➢
- NInChI supply information for simulation, and link input and output data and simulation parameters as part of an automated nanoinformatics workflow. All model data can be stored and linked together with the corresponding NInChI. This allows data retrieval based on specific NM queries. Simulation parameters will also be stored as meta-data for a given NM and modelling method (Figure 6). An example of the three components for the nanodescriptors calculations based on atomistic simulations is given in Table 2.
- ➢
- NM information encoded in the NInChI will enable grouping of NMs based on compositional and structural similarity, and enable generation of computational nanodescriptors to encode the whole NM.
3.5.3. Conclusions on Relevant Features of a NInChI
3.6. Case Study 6. NM Regulation
3.6.1. Objective
- RNs are often assigned purely on the basis of the chemical structure, which often leads to ambiguities due to multiple substances with different RNs corresponding to the same chemical structure. This is similar to the multiple to-be-registered substances that correspond to one reference substance defined in IUCLID6 [154].
- For NMs, there is the additional, more severe problem that no RNs exist for the different nanoforms, defined in EU chemicals legislation (Registration, Evaluation, Authorisation and Restriction of Chemicals or REACH) as NMs with the same core chemistry but different sizes, shapes, coatings. For these it is suggested that the bulk or micron-sized materials RN be used. ECHA are currently addressing this in IUCLID6 by specifying different assessment entities within a single substance registration.
- Commonly encountered mixtures of known or unknown composition and even whole classes of molecules like classes of enzymes receive a single RN.
3.6.2. Specific Use Cases—Nanoforms Concept and NInChI as Solution for Regulators
- Fitting the needs of the nanoforms concept of REACH and ECHA. According to REACH Regulation (Annex R.6-1, [40]), a nanoform is a form of a natural or manufactured substance containing particles, in an unbound state or as an aggregate or agglomerate where, in which 50% or more of the particles have one or more dimensions in the range 1–100 nm. This includes fullerenes, graphene flakes and single wall carbon nanotubes with one or more external dimensions below 1 nm. A set of similar nanoforms is a group of nanoforms for which hazard, exposure, and risk assessment can be performed jointly. A justification must be provided to show variations within these boundaries does not significantly affect hazard, exposure, and risk assessment of the similar nanoforms in the set. A nanoform can only belong to one set of similar nanoforms. ECHA developed a stepwise approach following the steps outlined by the OECD guidance on grouping of chemicals [156]. ECHA requires information on the nanoform that includes composition of the substance, impurities or additives, surface treatment and functionalization (chemical coating and surface treatment(s) applied to the particles). It also includes physical parameters such as size distribution, shape aspect ratio and other morphological characterization data, crystallinity, information on assembly structure including (e.g., shell-like structures or hollow structures, if appropriate), and specific surface area (e.g., porosity). The user has to measure each property (using a standardized protocol), report the method and the results in IUCLID [157]. A set of nanoforms can exhibit a range of values for each property provided that the range does not impact the nanoform’s risk. The regulation requires that at least 50% of particles (number distribution) is within the range of 1 to 100 nm, with further information on the particle size distribution (e.g., d10, d50, d90 values). The registrants must define the boundary defining the set of similar nanoforms, for example by specifying the minimum d10 and maximum d90. A set of nanoforms should exhibit similar dissolution rates, toxicokinetic behaviors, fate and bioavailability and ecotoxicological parameters.
- ➢
- By using a structural representation, rather than a chemistry-unaware substance identifier, in Tiers 1-3 (Figure 1) encoding composition, size/shape and surface coating of nanoforms, NInChI will support the differentiation of individual nanoforms (assessment entities) independent of their inclusion in identifier repositories controlled by third-party organizations like the CAS registry.
- NInChI as a solution for the regulatory needs—Moving from CAS to NInChI. CAS RNs are completely arbitrary, contain no intrinsic information, and are easily misused as there is no way to validate them other than locating them in a database. Case study 4 highlighted some of the challenges presented by NMs regarding identification and linking of datasets to the specific NMs, batch-to-batch variability, NM ageing and transformation, and integrating data sets with confidence in the absence of unambiguous identifiers. Thus, for NMs there is a clear need for a representation like NInChI to replace or augment the CAS RN, or other chemistry-unaware identifiers, to increase confidence in datasets used in weight of evidence, grouping, read across, and QSAR modelling approaches. Using a NInChI to validate datasets, by checking the consistency of the data for the object under investigation, would significantly boost the quality of QSAR and read-across models, and the confidence with which they can be applied in nanosafety. Thus, in terms of addressing regulatory needs, NInChI will enable:
- Distinguishing of different nanoforms for registration (importance of standardized identifiers and structural representations, unified data management processes, etc.). The tiers in Figure 1 have been mapped to the information requirements included in Annex R.6-1, including shape, size and surface coating considerations and, as such, NInChIs will be an important means to differentiate individual nanoforms. By adapting the MInChI extension that includes ranges, we envisage incorporating a set of nanoforms into a single NInChI by providing ranges within which specific NM properties can vary while their toxicities remain the same, thus providing boundaries for a set of NMs. While this is not included explicitly in the current examples, an extension in a subsequent iteration of the NInChI would be possible.
- ➢
- NMs information included in the NInChI will support grouping of NMs based on both compositional and structural properties.
- b.
- Read across and grouping using NInChI for predictions, as described in detail in case study 5. While QSARs are well established for small molecules, their acceptance for regulatory purposes is still limited, mainly due to dataset uncertainty and often poor documentation of models in the QSAR model report forms (QMRFs). NInChIs will make it straightforward to update existing QMRFs with the NInChIs of all NMs that were used as part of the training and test sets. Using the NInChIs will enable extraction of NM structural information from databases and visual presentation. This will aid expert evaluation and interpretation of QSAR models for grouping strategies, determine whether it is applicable to the NMs under evaluation, and allow independent assessment of predictions and structural similarity. The notation that describes NMs from the center outwards will also allow a simplified graphical representation of the NMs key elements, potentially as a 2 D representation of the 3 D structure, as shown schematically in Figure 7.
- ➢
- NM information included in the NInChI supports verification and validation of grouping hypotheses based on simplified visualization of chemical and structural information.
3.6.3. Conclusions on Relevant Features of a NInChI
3.7. NInChI—A Proposal for a Layered Approach for Uniquely Identifying NMs
- It should be a unique representation, where a specific NM is always represented by the same NInChI and a NInChI is always associated with the same NM or group of closely related NMs. The latter modifies the one-to-one relationship of InChIs described in the introduction by accepting the stochastic nature of many NMs, discussed in more detail below.
- The structure should be optimal for extraction of specific information by a computer but also by a trained person (i.e., should have a degree of human interpretability)
- It be compatible with other notations in the InChI universe, reusing concepts or extensions of these notations or even incorporating their complete representations as part of the NInChI.
- InChI, PInChI or MInChI to represent the chemical composition (without the leading version number)
- morphology layer (prefix m): abbreviations are used for specific morphologies, e.g., sp: sphere, sh: shell, ro: rod, tu: tube
- size layer (prefix s): specified in scientific notation in meters, e.g., 2x-9 where x can be r: radius, d: diameter, l: length, t: thickness, ranges can be given separated by “:”
- crystal layer (prefix k)
- chirality layer (prefix w for carbon nanotubes)
3.8. NInChI Alpha—Demonstration of Worked Examples of NMs InChIs
- Purity ≥ 99%
- Rutile form, or rutile with up to 5% anatase, with crystalline structure and physical appearance as clusters of spherical, needle, or lanceolate shapes
- Median particle size based on number size distribution ≥ 30 nm
- Coated with silica, hydrated silica, alumina, aluminium hydroxide, aluminium stearate, stearate, stearic acid, trimethoxycaprylylsilane, glycerin, dimethicone, dimethicone/methicone copolymer, simethicone;
3.9. Prototype of a NInChI Generation Service
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Lynch, I. NanoSafety Cluster Compendium of Projects. 2017. Available online: https://www.nanosafetycluster.eu/wp-content/uploads/NSC%20Outputs/Compendium/2017_NSC_Compendium.pdf?_t=1537124047 (accessed on 8 October 2020).
- Katalagarianakis, G. Nano-EHS Activity and Strategy in the EU. 2013. Available online: https://www.fooddrinkeurope.eu/uploads/events_documents/4._Katalangarianakis.pdf (accessed on 8 October 2020).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co.: London, UK, 1959. [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 8 October 2020).
- Vinken, M. 3Rs toxicity testing and disease modeling projects in the European Horizon 2020 research and innovation program. EXCLI J. 2020, 19, 775–784. [Google Scholar]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef]
- Roca, C.P.; Rallo, R.; Fernández, A.; Giralt, F.; Fernández, A. Chapter 6. Nanoinformatics for Safe-by-Design Engineered Nanomaterials. In Towards Efficient Designing of Safe Nanomaterials: Innovative Merge of Computational Approaches and Experimental Techniques; Leszczynski, J., Puzyn, T., Eds.; RSC Nanoscience and Nanotechnology: Cambridge, UK, 2012; pp. 89–107. [Google Scholar]
- Isigonis, P.; Afantitis, A.; Antunes, D.; Bartonova, A.; Beitollahi, A.; Bohmer, N.; Bouman, E.; Chaudhry, Q.; Cimpan, M.R.; Cimpan, E.; et al. Risk Governance of Emerging Technologies Demonstrated in Terms of its Applicability to Nanomaterials. Small 2020, 16, 2003303. [Google Scholar] [CrossRef]
- Winkler, D.A. Role of Artificial Intelligence and Machine Learning in Nanosafety. Small 2020, 16, 2001883. [Google Scholar] [CrossRef]
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Heller, S.R.; McNaught, A.; Stein, S.E.; Tchekhovskoi, D.; Pletnev, I. InChI—The worldwide chemical structure identifier standard. J. Cheminform. 2013, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, S.R.; McNaught, A.; Pletnev, I.; Stein, S.E.; Tchekhovskoi, D. InChI, the IUPAC International Chemical Identifier. J. Cheminform. 2015, 7, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Weiss, C.; Valsami-Jones, E. A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design NMs. Nano Today 2014, 9, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef] [PubMed]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Grethe, G.; Goodman, J.M.; Allen, C.H.G. International chemical identifier for reactions (RInChI). J. Cheminform. 2013, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Grethe, G.; Blanke, G.; Kraut, H.; Goodman, J.M. International chemical identifier for reactions (RInChI). J. Cheminform. 2018, 10, 22. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, J.; Nieab, Z. Symmetry-Breaking Synthesis of Multicomponent Nanoparticles. Acc. Chem. Res. 2019, 52, 1125–1133. [Google Scholar] [CrossRef]
- Madkour, M.; Bumajdad, A.; Al-Sagheer, F. To what extent do polymeric stabilizers affect nanoparticles characteristics? Adv. Colloid Interface Sci. 2019, 270, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-S.; Coley, C.W.; Mochigase, H.; Beech, H.K.; Wang, W.; Wang, Z.; Woods, E.; Craig, S.L.; Johnson, J.A.; Kalow, J.A.; et al. BigSMILES: A Structurally-Based Line Notation for Describing Macromolecules. ACS Central Sci. 2019, 5, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Drefahl, A. CurlySMILES: A chemical language to customize and annotate encodings of molecular and nanodevice structures. J. Cheminform. 2011, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.M.; McEwen, L.R.; Gedeck, P.; Bunin, B.A. Capturing mixture composition: An open machine-readable format for representing mixed substances. J. Cheminform. 2019, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Romero, G.; Moya, S.E. Chapter 4—Synthesis of Organic Nanoparticles. In Frontiers of Nanoscience; de la Fuente, J.M., Grazu, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 115–141. [Google Scholar]
- Frank, P.; Ottoboni, M. The Dose Makes the Poison: A Plain-Language Guide to Toxicology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Xiaoli, F.; Qiyue, C.; Weihong, G.; Yaqing, Z.; Chen, H.; Junrong, W.; Shao, L. Toxicology data of graphene-family nanomaterials: An update. Arch. Toxicol. 2020, 94, 1915–1939. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Knowles, J.C.; Kim, H.-W. Advances in nanoparticle development for improved therapeutics delivery: Nanoscale topographical aspect. J. Tissue Eng. 2019, 10, 2041731419877528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, M.; De Liguoro, M.; Franzago, E.; Baratella, D.; Vianellod, F. The surface reactivity of iron oxide nanoparticles as a potential hazard for aquatic environments: A study on Daphnia magna adults and embryos. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vassey, M.J.; Figueredo, G.P.; Scurr, D.J.; Vasilevich, A.S.; Vermeulen, S.; Carlier, A.; Luckett, J.; Beijer, N.R.M.; Williams, P.; Winkler, D.; et al. Immune Modulation by Design: Using Topography to Control Human Monocyte Attachment and Macrophage Differentiation. Adv. Sci. 2020, 7, 1903392. [Google Scholar] [CrossRef] [PubMed]
- Carenco, S. Describing inorganic nanoparticles in the context of surface reactivity and catalysis. Chem. Commun. 2018, 54, 6719–6727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface characterization of nanomaterials and nanoparticles: Important needs and challenging opportunities. J. Vac. Sci. Technol. A 2013, 31, 050820. [Google Scholar] [CrossRef]
- Svendsen, C.; Walker, L.A.; Matzke, M.; Lahive, E.; Harrison, S.; Crossley, A.; Park, B.; Lofts, S.; Lynch, I.; Vázquez-Campos, S.; et al. Key principles and operational practices for improved nanotechnology environmental exposure assessment. Nat. Nanotechnol. 2020, 15, 731–742. [Google Scholar] [CrossRef]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting their Toxicity to Daphnia Magna—A Call for Updating Toxicity Testing Policies. Proteomics 2020, 20, 1800412. [Google Scholar] [CrossRef] [Green Version]
- Sund, J.; Alenius, H.; Vippola, M.; Savolainen, K.; Puustinen, A. Proteomic Characterization of Engineered Nanomaterial–Protein Interactions in Relation to Surface Reactivity. ACS Nano 2011, 5, 4300–4309. [Google Scholar] [CrossRef]
- Khan, A.O.; Di Maio, A.; Guggenheim, E.J.; Chetwynd, A.J.; Pencross, D.; Tang, S.; Belinga-Desaunay, M.-F.A.; Thomas, S.G.; Rappoport, J.Z.; Lynch, I. Surface Chemistry-Dependent Evolution of the Nanomaterial Corona on TiO2 Nanomaterials Following Uptake and Sub-Cellular Localization. Nanomaterials 2020, 10, 401. [Google Scholar] [CrossRef] [Green Version]
- Warheit, D.B.; Reed, K.L.; Sayes, C.M. A role for nanoparticle surface reactivity in facilitating pulmonary toxicity and development of a base set of hazard assays as a component of nanoparticle risk management. Inhal. Toxicol. 2009, 21 (Suppl. S1), 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Hellack, B.; Wiemann, M.; Giusti, A.; Werle, K.; Haase, A.; Wohlleben, W. Nanomaterial categorization by surface reactivity: A case study comparing 35 materials with four different test methods. NanoImpact 2020, 19, 100234. [Google Scholar] [CrossRef]
- OECD. Physical-chemical decision framework to inform decisions for Risk Assessment of manufactured nanomaterials. In ENV/JM/MONO(2019)12; Series on the Safety of Manufactured Nanomaterials No. 90; OECD: Paris, France, 2019. [Google Scholar]
- Nel, A.E.; Parak, W.J.; Chan, W.C.W.; Xia, T.; Hersam, M.C.; Brinker, C.J.; Zink, J.I.; Pinkerton, K.E.; Baer, D.R.; Weiss, P.S. Where Are We Heading in Nanotechnology Environmental Health and Safety and Materials Characterization? ACS Nano 2015, 9, 5627–5630. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Appendix R.6-1: Recommendations for Nanomaterials Applicable to the Guidance on QSARs and Grouping of Chemicals. 2017. Available online: https://echa.europa.eu/documents/10162/13564/appendix_r_6-1_nano_caracal_en.pdf/a07d989c-99ec-d87f-0f4d-b198022f4744 (accessed on 8 October 2020).
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 263. [Google Scholar]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Losic, D.; Simovic, S. Self-ordered nanopore and nanotube platforms for drug delivery applications. Expert Opin. Drug Deliv. 2009, 6, 1363–1381. [Google Scholar] [CrossRef]
- Wang, S.; Kowal, T.J.; Marei, M.K.; Falk, M.M.; Jain, H. Nanoporosity Significantly Enhances the Biological Performance of Engineered Glass Tissue Scaffolds. Tissue Eng. Part A 2013, 19, 1632–1640. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Andres, R.P.; Averback, R.S.; Brown, W.L.; Brus, L.E.; Goddard, W.A.; Kaldor, A.; Louie, S.G.; Moscovits, M.; Peercy, P.S.; Riley, S.J.; et al. Research opportunities on clusters and cluster-assembled materials—A Department of Energy, Council on Materials Science Panel Report. J. Mater. Res. 1989, 4, 704–736. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering Substrate Topography at the Micro- and Nanoscale to Control Cell Function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takato, Y.; Benson, M.E.; Sen, S. Small nanoparticles, surface geometry and contact forces. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20170723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utembe, W.; Wepener, V.; Yu, I.J.; Gulumian, M. An assessment of applicability of existing approaches to predicting the bioaccumulation of conventional substances in nanomaterials. Environ. Toxicol. Chem. 2018, 37, 2972–2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavia, D.J.; Shon, Y.-S. Controlling Surface Ligand Density and Core Size of Alkanethiolate-Capped Pd Nanoparticles and Their Effects on Catalysis. Langmuir 2012, 28, 14502–14508. [Google Scholar] [CrossRef] [Green Version]
- Nasser, F.; Davis, A.; Valsami-Jones, E.; Lynch, I. Shape and Charge of Gold Nanomaterials Influence Survivorship, Oxidative Stress and Moulting of Daphnia magna. Nanomaterials 2016, 6, 222. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, Y. Understanding the adsorption of branched polyamine on surface of gold nanoparticles by molecular dynamics simulations. Surf. Interface Anal. 2016, 48, 1379–1383. [Google Scholar] [CrossRef]
- Papadiamantis, A.G.; Klaessig, F.C.; Exner, T.E.; Hofer, S.; Hofstaetter, N.; Himly, M.; Williams, M.A.; Doganis, P.; Hoover, M.D.; Afantitis, A.; et al. Metadata Stewardship in Nanosafety Research: Community-Driven Organisation of Metadata Schemas to Support FAIR Nanoscience Data. Nanomaterials 2020, 10, 2033. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.T.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef] [Green Version]
- Toropov, A.A.; Toropova, A.P.; Benfenati, E.; Gini, G.; Leszczynska, D.; Leszczynski, J. SMILES-based QSAR approaches for carcinogenicity and anticancer activity: Comparison of correlation weights for identical SMILES attributes. Anti-Cancer Agents Med. Chem. 2011, 11, 974–982. [Google Scholar] [CrossRef]
- Gentleman, D.J.; Chan, W.C.W. A Systematic Nomenclature for Codifying Engineered Nanostructures. Small 2009, 5, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Olsen, J.B.; Song, F.; Liu, R.; Guo, H.; Olsen, D.W.H.; Cohen, Y.; Emili, A.; Chan, W.C.W. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural Defects Play a Major Role in the Acute Lung Toxicity of Multiwall Carbon Nanotubes: Toxicological Aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Khalap, V.R.; Sheps, T.; Kane, A.A.; Collins, P.G. Hydrogen Sensing and Sensitivity of Palladium-Decorated Single-Walled Carbon Nanotubes with Defects. Nano Lett. 2010, 10, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Li, Y.; Yin, J.-J.; Liu, Y.; Wang, L.; Zhao, Y.; Chen, C. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4, e32. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Del Rosal, B.; Zhang, Y.; Rodríguez, E.M.; Hu, J.; Zhou, Z.; Fan, R.; Ortgies, D.H.; Fernández, N.; Chaves-Coira, I.; et al. Rare-earth-doped fluoride nanoparticles with engineered long luminescence lifetime for time-gated in vivo optical imaging in the second biological window. Nanoscale 2018, 10, 17771–17780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of Metal Oxide Nanoparticle Band Gap To Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Zadesenets, A.V.; Asanova, T.; Vikulova, E.; Filatov, E.; Plyusnin, P.E.; Baidina, I.; Asanov, I.; Korenev, S. Solid solutions of platinum(II) and palladium(II) oxalato-complex salt as precursors of nanoalloys. J. Solid State Chem. 2013, 199, 71–77. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for cancer therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Mori, T.; Hegmann, T. Determining the composition of gold nanoparticles: A compilation of shapes, sizes, and calculations using geometric considerations. J. Nanoparticle Res. 2016, 18, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Van der Zande, B.M.I.; Böhmer, M.R.; Fokkink, L.G.J.; Schönenberger, C. Aqueous Gold Sols of Rod-Shaped Particles. J. Phys. Chem. B 1997, 101, 852–854. [Google Scholar] [CrossRef]
- Ahmad, R.; Fu, J.; He, N.; Li, S. Advanced Gold Nanomaterials for Photothermal Therapy of Cancer. J. Nanosci. Nanotechnol. 2016, 16, 67–80. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lei, Q.; Luo, G.-F.; Jia, H.-Z.; Hong, S.; Liu, Y.-X.; Cheng, Y.-J.; Zhang, X.-Z. Rational Design of Multifunctional Gold Nanoparticles via Host–Guest Interaction for Cancer-Targeted Therapy. ACS Appl. Mater. Interfaces 2015, 7, 17171–17180. [Google Scholar] [CrossRef]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Meenan, B.J.; Mutreja, I.; D’Sa, R.; Dixon, D. Controlling the size and size distribution of gold nanoparticles: A design of EXPERIMENT study. Int. J. Nanosci. 2012, 11, 1250023. [Google Scholar] [CrossRef]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.-J. Validation of Size Estimation of Nanoparticle Tracking Analysis on Polydisperse Macromolecule Assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiber, C.; Kasper, J.C.; Milani, S.; Scheible, M.; Martín, I.; Schaubhut, F.; Küchler, S.; Rädler, J.; Simmel, F.C.; Friess, W.; et al. Comparison of four different particle sizing methods for siRNA polyplex characterization. Eur. J. Pharm. Biopharm. 2013, 84, 255–264. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Anderson, W.; Kozak, D.; Coleman, V.A.; Jämting, Å.K.; Trau, M. A comparative study of submicron particle sizing platforms: Accuracy, precision and resolution analysis of polydisperse particle size distributions. J. Colloid Interface Sci. 2013, 405, 322–330. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Zook, J.M.; MacCuspie, R.I.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef] [Green Version]
- Schavkan, A.; Gollwitzer, C.; Garcia-Diez, R.; Krumrey, M.; Minelli, C.; Bartczak, D.; Cuello-Nuñez, S.; Goenaga-Infante, H.; Rissler, J.; Sjöström, E.; et al. Number Concentration of Gold Nanoparticles in Suspension: SAXS and spICPMS as Traceable Methods Compared to Laboratory Methods. Nanomaterials 2019, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Wani, I.A.; Ahmed, J.; Al-Hartomy, O.A. Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 2014, 4, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Caruso, F.; Spasova, M.; Salgueiriño-Maceira, V.; Liz-Marzán, L.M. Multilayer Assemblies of Silica-Encapsulated Gold Nanoparticles on Decomposable Colloid Templates. Adv. Mater. 2001, 13, 1090–1094. [Google Scholar] [CrossRef]
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold Nanocages: Bioconjugation and Their Potential Use as Optical Imaging Contrast Agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; McLellan, J.M.; Siekkinen, A.; Xiong, Y.; Li, Z.-Y.; Xia, Y. Facile Synthesis of Gold−Silver Nanocages with Controllable Pores on the Surface. J. Am. Chem. Soc. 2006, 128, 14776–14777. [Google Scholar] [CrossRef] [Green Version]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [Green Version]
- Zalipsky, S. Chemistry of polyethylene glycol conjugates with biologically active molecules. Adv. Drug Deliv. Rev. 1995, 16, 157–182. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 410–422. [Google Scholar] [CrossRef]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Talirz, L.; Pignedoli, C.A.; Feng, X.; Muellen, K.; Fasel, R.; Ruffieux, P. Giant edge state splitting at atomically precise graphene zigzag edges. Nat. Commun. 2016, 7, 11507. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mu, F.; Wang, Y.; Zhao, H. Graphene and Graphene-Based Nanomaterials for DNA Detection: A Review. Molecules 2018, 23, 2050. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.; Bourlinos, A.B.; Kim, K.S.; Zbořil, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- Mazánek, V.; Luxa, J.; Matějková, S.; Kučera, J.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Ultrapure Graphene Is a Poor Electrocatalyst: Definitive Proof of the Key Role of Metallic Impurities in Graphene-Based Electrocatalysis. ACS Nano 2019, 13, 1574–1582. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohner, N.; Lam, P.K.; Sattler, K. Systematic energetics study of graphene nanoflakes: From armchair and zigzag to rough edges with pronounced protrusions and overcrowded bays. Carbon 2015, 82, 523–537. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yang, J. Chapter 2 Structure and Properties of Carbon Nanotubes. In Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–69. [Google Scholar]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun Doping of Carbon Nanotubes and Platinum Nanoparticles into the β-Phase Polyvinylidene Difluoride Nanofibrous Membrane for Biosensor and Catalysis Applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef]
- Arbab, A.A.; Memon, A.A.; Sahito, I.A.; Mengal, N.; Sun, K.C.; Ali, M.; Jeong, S.H. An evidence for an organic N-doped multiwall carbon nanotube heterostructure and its superior electrocatalytic properties for promising dye-sensitized solar cells. J. Mater. Chem. A 2018, 6, 8307–8322. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12–18. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Liu, K.; Cui, Y. Design of Complex Nanomaterials for Energy Storage: Past Success and Future Opportunity. Acc. Chem. Res. 2017, 50, 2895–2905. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Jing, X.; Pan, M.; Liu, P.; Li, W.; Zhu, B.; Li, J.; Chen, H.; Wang, L.; et al. Complex silica composite nanomaterials templated with DNA origami. Nature 2018, 559, 593–598. [Google Scholar] [CrossRef]
- Ellis, L.-J.A.; Papadiamantis, A.G.; Weigel, S.; Valsami-Jones, E. Synthesis and characterization of Zr- and Hf-doped nano-TiO2 as internal standards for analytical quantification of nanomaterials in complex matrices. R. Soc. Open Sci. 2018, 5, 171884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, J.G.; Cattoën, X.; Durand, J.-O.; Man, M.W.C.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.F.; Su Su Zin, A.K.; Chen, Z.; Liow, C.H.; Phan, H.T.; Tan, H.R.; Xu, Q.-H.; Ho, G.W. Inverse Stellation of CuAu-ZnO Multimetallic-Semiconductor Nanostartube for Plasmon-Enhanced Photocatalysis. ACS Nano 2018, 12, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Luo, Z.; Huang, X.; Li, B.; Chen, Y.; Wang, J.; Hu, Y.; Zhang, H. Synthesis of 4H/fcc Noble Multimetallic Nanoribbons for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2016, 138, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Rashad, M.; Ali, Z.; Qiu, H.; Li, W.; Pan, L.; Hou, Y. Ni-doped MnO2/CNT nanoarchitectures as a cathode material for ultra-long life magnesium/lithium hybrid ion batteries. Mater. Today Energy 2018, 10, 108–117. [Google Scholar] [CrossRef]
- Koklic, T.; Urbančič, I.; Zdovc, I.; Golob, M.; Umek, P.; Arsov, Z.; Dražić, G.; Pintarič, Š.; Dobeic, M.; Štrancar, J. Surface deposited one-dimensional copper-doped TiO2 nanomaterials for prevention of health care acquired infections. PLoS ONE 2018, 13, e0201490. [Google Scholar] [CrossRef]
- Benítez-Martínez, S.; López-Lorente, Á.I.; Valcárcel, M. Determination of TiO2 nanoparticles in sunscreen using N-doped graphene quantum dots as a fluorescent probe. Microchim. Acta 2015, 183, 781–789. [Google Scholar] [CrossRef]
- Saxena, V.; Chandra, P.; Pandey, L.M. Design and characterization of novel Al-doped ZnO nanoassembly as an effective nanoantibiotic. Appl. Nanosci. 2018, 8, 1925–1941. [Google Scholar] [CrossRef]
- Prechtl, M.H.G.; Campbell, P.S. Metal oxide and bimetallic nanoparticles in ionic liquids: Synthesis and application in multiphase catalysis. Nanotechnol. Rev. 2013, 2, 577–595. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, H.; Cui, L.; Ouyang, C.; Shi, S.; Tang, W.; Li, H.; Lee, J.-S.; Chen, L. First-principles investigation on redox properties of M-doped CeO2 (M = Mn,Pr,Sn,Zr). Phys. Rev. B 2010, 82, 125104. [Google Scholar] [CrossRef]
- Hume-Rothery, W. Atomic diameters, atomic volumes and solid solubility relations in alloys. Acta Met. 1966, 14, 17–20. [Google Scholar] [CrossRef]
- Goodman, D.; Bennett, L.; Watson, R. Valency effects and relative solubilities in transition metal alloys. Scr. Met. 1983, 17, 91–96. [Google Scholar] [CrossRef]
- Yabuta, H.; Tanaka, H.; Furuta, T.; Watanabe, T.; Kubota, M.; Matsuda, T.; Ifuku, T.; Yoneda, Y. Enhancement of tetragonal anisotropy and stabilisation of the tetragonal phase by Bi/Mn-double-doping in BaTiO3 ferroelectric ceramics. Sci. Rep. 2017, 7, 45842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeleye, A.S.; Pokhrel, S.; Mädler, L.; Keller, A.A. Influence of nanoparticle doping on the colloidal stability and toxicity of copper oxide nanoparticles in synthetic and natural waters. Water Res. 2018, 132, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Joonas, E.; Aruoja, V.; Olli, K.; Syvertsen-Wiig, G.; Vija, H.; Kahru, A. Potency of (doped) rare earth oxide particles and their constituent metals to inhibit algal growth and induce direct toxic effects. Sci. Total. Environ. 2017, 593–594, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Burello, E.; Worth, A.P. A theoretical framework for predicting the oxidative stress potential of oxide nanoparticles. Nanotoxicology 2011, 5, 228–235. [Google Scholar] [CrossRef]
- Lee, R.; Stokes, E. Twenty-first Century Novel: Regulating Nanotechnologies. J. Environ. Law 2009, 21, 469–482. [Google Scholar] [CrossRef]
- Virkutyte, J.; Al-Abed, S.R.; Dionysiou, D.D. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients. Chem. Eng. J. 2012, 191, 95–103. [Google Scholar] [CrossRef]
- Jacobsen, A.; de Miranda Azevedo, R.; Juty, N.; Batista, D.; Coles, S.; Cornet, R.; Courtot, M.; Crosas, M.; Dumontier, M.; Evelo, C.T.; et al. FAIR Principles: Interpretations and Implementation Considerations. Data Intell. 2020, 2, 10–29. [Google Scholar] [CrossRef]
- Totaro, S.; Cotogno, G.; Rasmussen, K.; Pianella, F.; Roncaglia, M.; Olsson, H.; Sintes, J.M.R.; Crutzen, H.P. The JRC Nanomaterials Repository: A unique facility providing representative test materials for nanoEHS research. Regul. Toxicol. Pharmacol. 2016, 81, 334–340. [Google Scholar] [CrossRef]
- Southan, C. InChI in the wild: An assessment of InChIKey searching in Google. J. Cheminform. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; De La Torre, J.G.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniak, A.B.; Hackley, V.A.; Roebben, G.; Ehara, K.; Hankin, S.; Postek, M.T.; Lynch, I.; Fu, W.-E.; Linsinger, T.P.; Thünemann, A.F. Nanoscale reference materials for environmental, health and safety measurements: Needs, gaps and opportunities. Nanotoxicology 2013, 7, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.M.; Lynch, I.; Peijnenburg, W.; Rumble, J.; Klaessig, F.; Marquardt, C.; Rauscher, H.; Puzyn, T.; Purian, R.; Åberg, C.; et al. How should the completeness and quality of curated nanomaterial data be evaluated? Nanoscale 2016, 8, 9919–9943. [Google Scholar] [CrossRef]
- Willighagen, E. European Registry of Materials. 2019. Available online: https://github.com/NanoCommons/identifiers (accessed on 8 October 2020).
- Papadiamantis, A.G.; Jänes, J.; Voyiatzis, E.; Sikk, L.; Burk, J.; Burk, P.; Tsoumanis, A.; Ha, M.K.; Yoon, T.H.; Valsami-Jones, E.; et al. Predicting cytotoxicity of metal oxide nanoparticles using Isalos Analytics platform. Nanomaterials 2020, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Afantitis, A.; Melagraki, G.; Isigonis, P.; Tsoumanis, A.; Varsou, D.D.; Valsami-Jones, E.; Papadiamantis, A.; Ellis, L.-J.A.; Sarimveis, H.; Doganis, P.; et al. NanoSolveIT Project: Driving nanoinformatics research to develop innovative and integrated tools for in silico nanosafety assessment. Comput. Struct. Biotechnol. J. 2020, 18, 583–602. [Google Scholar] [CrossRef]
- Ede, J.D.; Lobaskin, V.; Vogel, U.; Lynch, I.; Halappanavar, S.; Doak, S.H.; Roberts, M.G.; Shatkin, J.A. Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials. Nanomaterials 2020, 10, 1229. [Google Scholar] [CrossRef]
- Lamon, L.; Asturiol, D.; Vilchez, A.; Ruperez-Illescas, R.; Cabellos, J.; Richarz, A.; Worth, A. Computational models for the assessment of manufactured nanomaterials: Development of model reporting standards and mapping of the model landscape. Comput. Toxicol. 2019, 9, 143–151. [Google Scholar] [CrossRef]
- Forest, V.; Hochepied, J.-F.; Pourchez, J. Importance of Choosing Relevant Biological End Points To Predict Nanoparticle Toxicity with Computational Approaches for Human Health Risk Assessment. Chem. Res. Toxicol. 2019, 32, 1320–1326. [Google Scholar] [CrossRef]
- Afantitis, A.; Tsoumanis, A.; Melagraki, G. Enalos Suite of tools: Enhance Cheminformatics and Nanoinformatics through KNIME. Curr. Med. Chem. 2020, 27, 6523–6535. [Google Scholar] [CrossRef]
- Varsou, D.; Afantitis, A.; Tsoumanis, A.; Papadiamantis, A.; Valsami-Jones, E.; Lynch, I.; Melagraki, G. Zeta-Potential Read-Across Model Utilizing Nanodescriptors Extracted via the NanoXtract Image Analysis Tool Available on the Enalos Nanoinformatics Cloud Platform. Small 2020, 16, e1906588. [Google Scholar] [CrossRef] [PubMed]
- OECD. Customisation Opportunities of IUCLID6 for the Management of Chemical Data. In OECD Series on Testing and Assessment; OECD: Paris, France, 2019; Volume 297. [Google Scholar]
- CAS. Available online: https://www.cas.org/support/documentation/chemical-substances/faqs#2 (accessed on 24 October 2020).
- OECD. Guidance on Grouping of Chemicals, Second Edition. In OECD Series on Testing and Assessment; OECD: Paris, France, 2017. [Google Scholar]
- Rauscher, H.; Roebben, G.; Mech, A.; Gibson, N.; Kestens, V.; Linsinger, T.P.J.; Riego Sintes, J. An Overview of Concepts and Terms Used in the European Commission’s Definition of Nanomaterial; European Commisson: Brussels, Belgium, 2019. [Google Scholar]
- SCCS. Opinion on Titanium Dioxide (Nano Form) as UV-Filterin sprays. SCCS/1583/17. 2018. Available online: http://publications.europa.eu/resource/cellar/b635a200-38cd-11e9-8d04-01aa75ed71a1.0001.01/DOC_1 (accessed on 8 October 2020).
- Zhao, Q.; Zhang, J. Characterizing the Chiral Index of a Single-Walled Carbon Nanotube. Small 2014, 10, 4586–4605. [Google Scholar] [CrossRef] [PubMed]









| Required Simulation Input | Information Encoded in the NInChI |
|---|---|
| Core material chemistry | Au (Gold) |
| Size | 20d-9 (20 nm) |
| Shape | sp (sphere) |
| NInChI | Simulations Input | Simulations Output |
|---|---|---|
| Core/size/shape/polyform | The structure (i.e., coordinates of all atoms) of a NM and input parameters (Buckingham and Coulomb force field parameterization) | Structural and energetic descriptors of the NM |
| Category 1: Must Have | Category 2a: Nice to Have | Category 2b: Extrinsic Properties | Category 3: Out of Scope |
|---|---|---|---|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynch, I.; Afantitis, A.; Exner, T.; Himly, M.; Lobaskin, V.; Doganis, P.; Maier, D.; Sanabria, N.; Papadiamantis, A.G.; Rybinska-Fryca, A.; et al. Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies? Nanomaterials 2020, 10, 2493. https://doi.org/10.3390/nano10122493
Lynch I, Afantitis A, Exner T, Himly M, Lobaskin V, Doganis P, Maier D, Sanabria N, Papadiamantis AG, Rybinska-Fryca A, et al. Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies? Nanomaterials. 2020; 10(12):2493. https://doi.org/10.3390/nano10122493
Chicago/Turabian StyleLynch, Iseult, Antreas Afantitis, Thomas Exner, Martin Himly, Vladimir Lobaskin, Philip Doganis, Dieter Maier, Natasha Sanabria, Anastasios G. Papadiamantis, Anna Rybinska-Fryca, and et al. 2020. "Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies?" Nanomaterials 10, no. 12: 2493. https://doi.org/10.3390/nano10122493
APA StyleLynch, I., Afantitis, A., Exner, T., Himly, M., Lobaskin, V., Doganis, P., Maier, D., Sanabria, N., Papadiamantis, A. G., Rybinska-Fryca, A., Gromelski, M., Puzyn, T., Willighagen, E., Johnston, B. D., Gulumian, M., Matzke, M., Green Etxabe, A., Bossa, N., Serra, A., ... Melagraki, G. (2020). Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies? Nanomaterials, 10(12), 2493. https://doi.org/10.3390/nano10122493