Direct Laser Writing of Transparent Polyimide Film for Supercapacitor
Abstract
:1. Introduction
2. Experiment
2.1. Preparation and Characterizations
2.2. Electrochemical Measurements
3. Results and Discussions
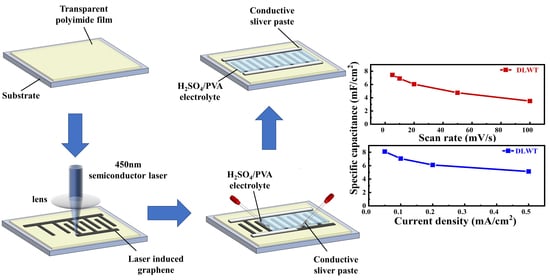
3.1. Characteristics of Materials
3.2. Electrochemical Performance of the Supercapacitors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, B.Z.; Liu, C.; Neff, D.; Yu, Z.; Wang, M.C.; Xiong, W.; Zhamu, A. Graphene surface-enabled lithium ion-exchanging cells: Next-generation high-power energy storage devices. Nano Lett. 2011, 11, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, J.; Noh, J.; Lim, N.; Lim, C.; Lee, G.; Kim, J.; Kang, H.; Jung, K.; Leonard, A.D.; et al. All-printed and roll-to-roll-printable 13.56-MHz-operated 1-bit RF tag on plastic foils. IEEE Trans. Electron Devices 2010, 57, 571–580. [Google Scholar] [CrossRef]
- Phan, H.P.; Dinh, T.; Nguyen, T.K.; Qamar, A.; Nguyen, T.; Dau, V.T.; Han, J.; Dao, D.V.; Nguyen, N.T. High temperature silicon-carbide-based flexible electronics for monitoring hazardous environments. J. Hazard. Mater. 2020, 394, 122486. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible electronics toward wearable sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, S.; Vorobyev, A.; Gamstedt, K.; Wu, K.; Guo, C.; Jeong, S.H. Seamless modulus gradient structures for highly resilient, stretchable system integration. Mater. Today Phys. 2018, 4, 28–35. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Jiang, H.B.; Shao, R.Q.; Zhang, Y.L.; Xie, S.Y.; Wang, J.N.; Li, X.B.; Jiang, F.; Chen, Q.D.; Zhang, T.J.C. Two-beam-laser interference mediated reduction, patterning and nanostructuring of graphene oxide for the production of a flexible humidity sensing device. Carbon 2012, 50, 1667–1673. [Google Scholar] [CrossRef]
- Strong, V.; Dubin, S.; El-Kady, M.F.; Lech, A.; Wang, Y.; Weiller, B.H.; Kaner, R.B. Patterning and electronic tuning of laser scribed graphene for flexible all-carbon devices. ACS Nano 2012, 6, 1395–1403. [Google Scholar] [CrossRef]
- Tian, H.; Shu, Y.; Cui, Y.L.; Mi, W.T.; Yang, Y.; Xie, D.; Ren, T.-L. Scalable fabrication of high-performance and flexible graphene strain sensors. Nanoscale 2013, 6, 699–705. [Google Scholar] [CrossRef]
- Tian, H.; Yang, Y.; Xie, D.; Cui, Y.-L.; Mi, W.-T.; Zhang, Y.; Ren, T.-L. Wafer-scale integration of graphene-based electronic, optoelectronic and electroacoustic devices. Sci. Rep. 2014, 4, 3598. [Google Scholar] [CrossRef] [Green Version]
- Luongo, G.; Di Bartolomeo, A.; Giubileo, F.; Chavarin, C.A.; Wenger, C. Electronic properties of graphene/p-silicon Schottky junction. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Singh, R.S.; Nalla, V.; Chen, W.; Wee, A.T.S.; Ji, W. Laser patterning of epitaxial graphene for schottky junction photodetectors. ACS Nano 2011, 5, 5969–5975. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A.; Luongo, G.; Iemmo, L.; Urban, F.; Giubileo, F. Graphene–silicon schottky diodes for photodetection. IEEE Trans. Nanotechnol. 2018, 17, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Chyan, Y.; Zhang, J.; Li, Y.; Han, X.; Kittrell, C.; Tour, J.M. Laser-induced graphene formation on wood. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Luo, S.; Cheng, X.; Feng, G.; Zhang, Y.; Wu, Z.; Compagnini, G.; Pooran, J.; Hu, A. All-solid-state supercapacitors from natural lignin-based composite film by laser direct writing. Appl. Phys. Lett. 2019, 115. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light laser-induced graphene from phenolic resin: A new approach for directly writing graphene-based electrochemical devices on various substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.; Ding, H.; Pan, N.; Wang, X. Fabrication of low-cost and highly sensitive graphene-based pressure sensors by direct laser scribing polydimethylsiloxane. ACS Appl. Mater. Interfaces 2019, 11, 6195–6200. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, D.; Wang, K.; Dong, X.; Duan, W.; Wang, F.; Gao, M.; Zhang, G. Direct laser writing of graphene films from a polyether ether ketone precursor. J. Mater. Sci. 2018, 54, 4192–4201. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-doped laser-induced porous graphene derived from polysulfone-class polymers and membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef]
- Ye, R.; Han, X.; Kosynkin, D.V.; Li, Y.; Zhang, C.; Jiang, B.; Marti, A.A.; Tour, J.M. Laser-induced conversion of teflon into fluorinated nanodiamonds or fluorinated graphene. ACS Nano 2018, 12, 1083–1088. [Google Scholar] [CrossRef]
- Samouco, A.; Marques, A.C.; Pimentel, A.; Martins, R.; Fortunato, E.J.F.; Electronics, P. Laser-induced electrodes towards low-cost flexible UV ZnO sensors. Flex. Print. Electron. 2018, 3, 044002. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.E.; Long, S.; Luo, Y.; Yu, P.; Tan, Z.; Bai, J.; Qu, B.; Yang, Y.; Shi, J.; et al. Three-dimensional printing of polyaniline/reduced graphene oxide composite for high-performance planar supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 10437–10444. [Google Scholar] [CrossRef]
- Ciesielski, A.; Haar, S.; Aliprandi, A.; El Garah, M.; Tregnago, G.; Cotella, G.F.; El Gemayel, M.; Richard, F.; Sun, H.; Cacialli, F.J.A.N. Modifying the size of ultrasound-induced liquid-phase exfoliated graphene: From nanosheets to nanodots. ACS Nano 2016, 10, 10768–10777. [Google Scholar] [CrossRef] [Green Version]
- Munuera, J.M.; Paredes, J.I.; Enterría, M.; Pagán, A.; Villar-Rodil, S.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L.; Cenis, J.L.; Martínez-Alonso, A.; et al. Electrochemical exfoliation of graphite in aqueous sodium halide electrolytes toward low oxygen content graphene for energy and environmental applications. ACS Appl. Mater. Interfaces 2017, 9, 24085–24099. [Google Scholar] [CrossRef]
- Thodkar, K.; Thompson, D.; Lüönd, F.; Moser, L.; Overney, F.; Marot, L.; Schönenberger, C.; Jeanneret, B.; Calame, M. Restoring the electrical properties of CVD graphene via physisorption of molecular adsorbates. ACS Appl. Mater. Interfaces 2017, 9, 25014–25022. [Google Scholar] [CrossRef]
- Momeni Pakdehi, D.; Aprojanz, J.; Sinterhauf, A.; Pierz, K.; Kruskopf, M.; Willke, P.; Baringhaus, J.; Stöckmann, J.P.; Traeger, G.A.; Hohls, F.; et al. Minimum resistance anisotropy of epitaxial graphene on SiC. ACS Appl. Mater. Interfaces 2018, 10, 6039–6045. [Google Scholar] [CrossRef]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2010, 16, 203–213. [Google Scholar] [CrossRef]
- Sun, G.; An, J.; Chua, C.K.; Pang, H.; Zhang, J.; Chen, P. Layer-by-layer printing of laminated graphene-based interdigitated microelectrodes for flexible planar micro-supercapacitors. Electrochem. Commun. 2015, 51, 33–36. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C.J.C. Carbonaceous nanomaterials employed in the development of electrochemical sensors based on screen-printing technique—A review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.S.; Parvez, K.; Winter, A.; Vieker, H.; Liu, X.; Han, S.; Turchanin, A.; Feng, X.; Mullen, K. Layer-by-layer assembled heteroatom-doped graphene films with ultrahigh volumetric capacitance and rate capability for micro-supercapacitors. Adv. Mater. 2014, 26, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- In, J.B.; Hsia, B.; Yoo, J.-H.; Hyun, S.; Carraro, C.; Maboudian, R.; Grigoropoulos, C.P. Facile fabrication of flexible all solid-state micro-supercapacitor by direct laser writing of porous carbon in polyimide. Carbon 2015, 83, 144–151. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Li, R.; Feng, G.; Wu, Z.; Compagnini, G.; Gulino, A.; Feng, Z.; Hu, A. High-performance stacked in-plane supercapacitors and supercapacitor array fabricated by femtosecond laser 3D direct writing on polyimide sheets. Electrochim. Acta 2017, 241, 153–161. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Pérez del Pino, Á.; Martínez Villarroya, A.; Chuquitarqui, A.; Logofatu, C.; Tonti, D.; György, E. Reactive laser synthesis of nitrogen-doped hybrid graphene-based electrodes for energy storage. J. Mater. Chem. A 2018, 6, 16074–16086. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-K.; Lin, Z.; Li, L.; Song, B.; Moon, K.-S.; Bai, S.-L.; Wong, C.-P. Flexible micro-supercapacitor based on in-situ assembled graphene on metal template at room temperature. Nano Energy 2014, 10, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Cai, J.; Lv, C.; Watanabe, A. Cost-effective fabrication of high-performance flexible all-solid-state carbon micro-supercapacitors by blue-violet laser direct writing and further surface treatment. J. Mater. Chem. A 2016, 4, 1671–1679. [Google Scholar] [CrossRef]
- Lamberti, A.; Perrucci, F.; Caprioli, M.; Serrapede, M.; Fontana, M.; Bianco, S.; Ferrero, S.; Tresso, E. New insights on laser-induced graphene electrodes for flexible supercapacitors: Tunable morphology and physical properties. Nanotechnology 2017, 28, 174002. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 decoration of laser-induced graphene as flexible supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Feng, G.; Yin, J.; Zhou, S.; Shen, L.; Wang, S.; Luo, Y. Direct Laser Writing of Transparent Polyimide Film for Supercapacitor. Nanomaterials 2020, 10, 2547. https://doi.org/10.3390/nano10122547
Huang F, Feng G, Yin J, Zhou S, Shen L, Wang S, Luo Y. Direct Laser Writing of Transparent Polyimide Film for Supercapacitor. Nanomaterials. 2020; 10(12):2547. https://doi.org/10.3390/nano10122547
Chicago/Turabian StyleHuang, Fei, Guoying Feng, Jiajia Yin, Sikun Zhou, Li Shen, Shutong Wang, and Yun Luo. 2020. "Direct Laser Writing of Transparent Polyimide Film for Supercapacitor" Nanomaterials 10, no. 12: 2547. https://doi.org/10.3390/nano10122547
APA StyleHuang, F., Feng, G., Yin, J., Zhou, S., Shen, L., Wang, S., & Luo, Y. (2020). Direct Laser Writing of Transparent Polyimide Film for Supercapacitor. Nanomaterials, 10(12), 2547. https://doi.org/10.3390/nano10122547