A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Anatase TiO2 NPs (TNP) and Ag-TiO2 NPs (Ag-TNP)
2.2.2. Preparation of Nanocomposites for SLA 3D Printing
2.2.3. Fabrication of Three-Dimensional Structures by SLA 3D Printer
2.3. Measurements and Characterization
3. Results and Discussion
3.1. Characterization of Ag-TiO2 Nanoparticles (Ag-TNP)
3.2. Characterization of SLR/Ag-TNP Nanocomposites
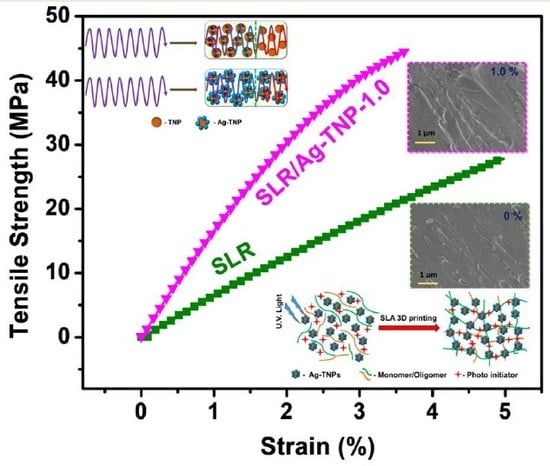
3.2.1. Mechanical Properties
3.2.2. Thermal Properties
3.3. Thermal and Mechanical Reinforcement Mechanism of SLR/Ag-TNP 3D Printed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired Detoxification Using 3D-Printed Hydrogel Nanocomposites. Nat. Commun. 2014, 8, 3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Valk, D.C.; van der Ven, C.F.T.; Blaser, M.C.; Grolman, J.M.; Wu, P.J.; Fenton, O.S.; Lee, L.H.; Tibbitt, M.W.; Andresen, J.L.; Wen, J.R.; et al. Engineering a 3D-bioprinted model of human heart valve disease using nanoindentation-based biomechanics. Nanomaterials 2018, 8, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, J.F.; Krull, B.P.; Garg, M.; Mangun, C.L.; Moore, J.S.; Sottos, N.R.; White, S.R. Robust sacrificial polymer templates for 3D interconnected microvasculature in fiber-reinforced composites. Compos. Pt. A Appl. Sci. Manuf. 2017, 100, 361–370. [Google Scholar] [CrossRef]
- Kamat, A.M.; Pei, Y.; Kottapalli, A.G.P. Bioinspired Cilia Sensors with Graphene Sensing Elements Fabricated Using 3D Printing and Casting. Nanomaterials 2019, 9, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H.; Lee, B.; Koo, J. Laser-induced graphene on additive manufacturing parts. Nanomaterials 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Shi, H.; Li, G.; Xue, Q.; Zhao, L.; Wang, F.; Hu, B. 3D-printed concentration-controlled microfluidic chip with diffusion mixing pattern for the synthesis of alginate drug delivery microgels. Nanomaterials 2019, 9, 1451. [Google Scholar] [CrossRef] [Green Version]
- Palmero, E.M.; Casaleiz, D.; de Vicente, J.; Hernández-Vicen, J.; López-Vidal, S.; Ramiro, E.; Bollero, A. Composites based on metallic particles and tuned filling factor for 3D-printing by Fused Deposition Modeling. Compos. Pt. A Appl. Sci. Manuf. 2019, 124, 105497. [Google Scholar] [CrossRef]
- Sugiyama, K.; Matsuzaki, R.; Ueda, M.; Todoroki, A.; Hirano, Y. 3D printing of composite sandwich structures using continuous carbon fiber and fiber tension. Compos. Pt. A Appl. Sci. Manuf. 2018, 113, 114–121. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, Z.; Huang, L.; Essa, K.; Bilotti, E.; Zhang, H.; Peijs, T.; Hao, L. Additive manufacturing high performance graphene-based composites: A review. Compos. Pt. A Appl. Sci. Manuf. 2019, 124, 105483. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Wang, Z.; Zhang, J.; Wang, Y. Multimaterial 3D Printing for Arbitrary Distribution with Nanoscale Resolution. Nanomaterials 2019, 9, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bártolo, P.J. Stereolithography: Materials, Processes and Applications, 1st ed.; Springer: New York, NY, USA, 2011; pp. 37–52. [Google Scholar]
- Fantino, E.; Vitale, A.; Quaglio, M.; Cocuzza, M.; Pirri, C.F.; Bongiovanni, R. Blue and UV combined photolithographic polymerization for the patterning of thick structures. Chem. Eng. J. 2015, 267, 65–72. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Purtov, J.; Rogin, P.; Verch, A.; Johansen, V.E. Nanopillar Di ff raction Gratings by Two-Photon Lithography. Nanomaterials 2019, 9, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2013, 26, 533–548. [Google Scholar] [CrossRef]
- Liu, Y.; Campbell, J.H.; Stein, O.; Jiang, L.; Hund, J.; Lu, Y. Deformation behavior of foam laser targets fabricated by two-photon polymerization. Nanomaterials 2018, 8, 498. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Shi, W. UV-cured organic–inorganic hybrid nanocomposite initiated by trimethoxysilane-modified fragmental photoinitiator. Compos. Pt. A Appl. Sci. Manuf. 2011, 42, 631–638. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Straub, M.; Gu, M. Acrylate-based photopolymer for two-photon micro-fabrication and photonic applications. Adv. Funct. Mater. 2005, 15, 209–216. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, T.L.; Lin, W.C.; Lin, H.Y.; Lee, M.H.; Yang, C.H. Crosslinked polymer ionic liquid/ionic liquid blends prepared by photopolymerization as solid-state electrolytes in supercapacitors. Nanomaterials 2018, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.Y.; Park, M.S.; Lee, J.W.; Yun, J.S. A study on the rheological and mechanical properties of photo-curable ceramic/polymer composites with different silane coupling agents for SLA 3D printing technology. Nanomaterials 2018, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappone, A.; Roppolo, I.; Naretto, E.; Fantino, E.; Calignano, F.; Sangermano, M.; Pirri, F. Study of graphene oxide-based 3D printable composites: Effect of the in situ reduction. Compos. Pt. B Eng. 2017, 124, 9–15. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Mangadlao, J.D.; Tiu, B.D.; Tritchler, G.C.; Advincula, R.C. High-strength Stereolithographic 3D printed nanocomposites: Graphene oxide meta-stability. ACS Appl. Mater. Interfaces 2017, 9, 10085–10093. [Google Scholar] [CrossRef]
- Gardner, J.M.; Sauti, G.; Kim, J.W.; Cano, R.J.; Wincheski, R.A.; Stelter, C.J.; Grimsley, B.W.; Working, D.C.; Siochi, E.J. 3-D printing of multifunctional carbon nano-tube yarn reinforced components. Addit. Manuf. 2016, 12, 38–44. [Google Scholar]
- Chen, S.G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK Reinforced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Tian, X.; Zhang, M.; Abliz, D.; Li, D.; Ziegmann, G. Interfacial performance and fracture patterns of 3D printed continuous carbon fiber with sizing reinforced PA6 composites. Compos. Pt. A Appl. Sci. Manuf. 2018, 114, 368–376. [Google Scholar] [CrossRef]
- Dalaq, A.S.; Abueidda, D.W.; Al-Rub, R.K.A. Mechanical properties of 3D printed interpenetrating phase composites with novel architectured 3D solid-sheet reinforcements. Compos. Pt. A Appl. Sci. Manuf. 2016, 84, 266–280. [Google Scholar] [CrossRef]
- Cano, M.; Khan, U.; Sainsbury, T.; O’Neill, A.; Wang, Z.; McGovern, I.T.; Maser, W.K.; Benito, A.M.; Coleman, J.N. Improving the mechanical properties of graphene oxide based materials by covalent attachment of polymer chains. Carbon 2013, 52, 363–371. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Guo, Y.; Zhang, H.; Zhang, Z. Improved thermal conductivity of thermoplastic polyurethane via aligned boron nitride platelets assisted by 3D printing. Compos. Pt. A Appl. Sci. Manuf. 2019, 120, 140–146. [Google Scholar] [CrossRef]
- Benad, A.; Jürries, F.; Vetter, B.; Klemmed, B.; Hübner, R.; Leyens, C.; Eychmüller, A. Mechanical properties of metal oxide aerogels. Chem. Mater. 2017, 30, 145–152. [Google Scholar] [CrossRef]
- Jia, Q.; Ghoshal, S.; Li, J.; Liang, W.; Meng, G.; Che, H.; Zhang, S.; Ma, Z.F.; Mukerjee, S. Metal and metal oxide interactions and their catalytic consequences for oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 7893–7903. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Chen, C.W. Polymer–metal-oxide hybrid solar cells. J. Mater. Chem. A 2013, 1, 10574–10591. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Hu, Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci. Process. Impacts 2012, 15, 39–48. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- .’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991, 353, 737. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterial’s: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, S.W. One-step hydrothermal synthesis of meso-porous anatase TiO2 microsphere and interfacial control for enhanced lithium storage performance. ACS Appl. Mater. Interfaces 2011, 3, 3697–3703. [Google Scholar] [CrossRef]
- Hao, B.; Yan, Y.; Wang, X.; Chen, G. Synthesis of anatase TiO2 nanosheets with enhanced pseudo capacitive contribution for fast lithium storage. ACS Appl. Mater. Interfaces 2013, 5, 6285–6291. [Google Scholar] [CrossRef]
- Giannuzzi, R.; Manca, M.; De Marco, L.; Belviso, M.R.; Cannavale, A.; Sibillano, T.; Giannini, C.; Cozzoli, P.D.; Gigli, G. Ultrathin TiO2 (B) nano-rods with superior lithium-ion storage performance. ACS Appl. Mater. Interfaces 2014, 6, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Zhu, H.; Zhang, D.; Chen, J. Elongated TiO2 nanotubes directly grown on graphene nanosheets as an efficient material for supercapacitors and absorbents. Compos. Pt. A Appl. Sci. Manuf. 2017, 10, 297–305. [Google Scholar] [CrossRef]
- Pawar, A.A.; Halivni, S.; Waiskopf, N.; Ben-Shahar, Y.; Soreni-Harari, M.; Bergbreiter, S.; Banin, U.; Magdassi, S. Rapid three-dimensional printing in water using semiconductor–metal hybrid nanoparticles as photoinitiators. Nano Lett. 2017, 17, 4497–4501. [Google Scholar] [CrossRef] [Green Version]
- Waiskopf, N.; Ben-Shahar, Y.; Galchenko, M.; Carmel, I.; Moshitzky, G.; Soreq, H.; Banin, U. Photocatalytic reactive oxygen species formation by semiconductor–metal hybrid nanoparticles. Toward light-induced modulation of biological processes. Nano Lett. 2016, 16, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Mathias, F.; Tahir, M.N.; Tremel, W.; Zentel, R. Functionalization of TiO2 Nanoparticles with Semiconducting Polymers Containing a Photo cleavable Anchor Group and Separation via Irradiation Afterward. Macromol. Chem. Phys. 2014, 215, 604–613. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef]
- Glass, S.; Trinklein, B.; Abel, B.; Schulze, A. TiO2 as Photosensitizer and Photoinitiator for Synthesis of Photoactive TiO2-PEGDA Hydrogel without Organic Photoinitiator. Front. Chem. 2018, 6, 340. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of TiO2-ZrO2 and thiol-acrylate resin nanocomposites with high refractive index via UV-induced crosslinking polymerization. Compos. Pt. A Appl. Sci. Manuf. 2007, 38, 1996–2004. [Google Scholar] [CrossRef]
- Prasad, V.; Sekar, K.; Varghese, S.; Joseph, M.A. Enhancing Mode I and Mode II interlaminar fracture toughness of flax fiber reinforced epoxy composites with nano TiO2. Compos. Pt. A Appl. Sci. Manuf. 2019, 124, 105505. [Google Scholar] [CrossRef]
- Thomas, S.P.; Thomas, S.; Bandyopadhyay, S. Mechanical, atomic force microscopy and focused ion beam studies of isotactic polystyrene/titanium dioxide composites. Compos. Pt. A Appl. Sci. Manuf. 2009, 40, 36–44. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core-shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag-TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Wang, S.; Han, Z.; Di, T.; Li, R.; Liu, S.; Cheng, Z. Preparation of pod-shaped TiO2 and Ag@TiO2 nano burst tubes and their photocatalytic activity. R. Soc. Open Sci. 2019, 6, 191019. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, L.; Gao, C.; Cao, L. The synthesis of nano-sized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar] [CrossRef]
- Ko, S.; Banerjee, C.K.; Sankar, J. Photochemical synthesis and photocatalytic activity in simulated solar light of nanosized Ag doped TiO2 nanoparticles composite. Compos. Pt. B Eng. 2011, 42, 579–583. [Google Scholar] [CrossRef]
- Yugang, D.; Yuan, Z.; Yiping, T.; Dichen, L. Nano-TiO2-modified photosensitive resin for RP. Rapid Prototyp. J 2011, 17, 247–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Li, Y.; Zhang, D.; Chen, Y. One-step synthesis of Ag@TiO2 nanoparticles for enhanced photocatalytic performance. Nanomaterials 2018, 8, 1032. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.; Li, X.; Wang, C. Plasmonic Ag-TiO2-X nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B Environ. 2017, 204, 67–77. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S. Photocatalytic degradation of methyl orange by CeO2 and Fe–doped CeO2 films under visible light irradiation. Sci Rep. 2014, 4, 5757. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of hetero-structure NiO/TiO2 nanomaterial’s used as efficient hetero-junction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.G. Transport characteristics of suspension: VIII. A note on the viscosity of Newtonian suspensions of uniform spherical particles. J. Colloid Sci. 1965, 20, 267–277. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Wu, L.; Weng, Z.; Zheng, L.; Peng, S.; Zhang, X. High performance POSS filled nanocomposites prepared via UV-curing based on 3D stereolithography printing. Compos. Pt. A Appl. Sci. Manuf. 2019, 117, 276–286. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, Y.; Lin, W.; Senthil, T.; Wu, L. Structure-property relationship of nano enhanced stereolithography resin for desktop SLA 3D printer. Compos. Pt. A Appl. Sci. Manuf. 2016, 88, 234–242. [Google Scholar] [CrossRef]
- Bahadur, S.; Henkin, A. Investigation of the ductility of rolled polymers. Polym. Eng. Sci. 1973, 13, 422–428. [Google Scholar] [CrossRef]
- Markandan, K.; Lai, C.Q. Enhanced mechanical properties of 3D printed graphene-polymer composite lattices at very low graphene concentrations. Compos. Pt. A Appl. Sci. Manuf. 2020, 129, 105726. [Google Scholar] [CrossRef]
- Amin, K.A. Reinforced materials based on chitosan, TiO2 and Ag composites. Polymers 2012, 4, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Harifi, T.; Montazer, M. Photo-, bio-, and magneto-active colored polyester fabric with hydrophobic/hydrophilic and enhanced mechanical properties through synthesis of TiO2/Fe3O4/Ag nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 1119–1129. [Google Scholar] [CrossRef]
- Senthil, T.; Divakaran, N.; Wang, J.; Wang, R.; Wu, L. Evolution of structural, electrical, and mechanical response of 3D robust network and conducting mechanically modified glass fabric-polyester composites with devisable 1D VGCNF. Compos. Sci. Technol. 2018, 163, 171–179. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, S.; Nie, K. Epoxy nanocomposites with octa(propylglycidyl ether) polyhedral oligomeric silsesquioxane. Polymer 2005, 46, 12016–12025. [Google Scholar] [CrossRef]
- Batmunkh, M.; Tanshen, M.R.; Nine, M.J.; Myekhlai, M.; Choi, H.; Chung, H.; Jeong, H. Thermal conductivity of TiO2 nanoparticles based aqueous nano-fluids with an addition of a modified silver particle. Ind. Eng. Chem. Res. 2014, 53, 8445–8451. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Kale, M.B.; Senthil, T.; Wu, L.; Wang, J. Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles. Nanomaterials 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Goldin, N.; Dodiuk, H.; Lewitus, D. Enhanced thermal conductivity of photopolymerizable composites using surface modified hexagonal boron nitride fillers. Compos. Sci. Technol. 2017, 152, 36–45. [Google Scholar] [CrossRef]
- Sangermano, M.; Calvara, L.; Chiavazzo, E.; Ventola, L.; Asinari, P.; Mittal, V.; Rizzoli, R.; Ortolani, L.; Morandi, V. Enhancement of electrical and thermal conductivity of Su-8 photocrosslinked coatings containing graphene. Prog. Org. Coat. 2015, 86, 143–146. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Graham, G.; Barandiaran, I.; Kortaberria, G. Electrically insulating polymeric nanocomposites with enhanced thermal conductivity by visible-light curing of epoxy–boron nitride nanotube formulations. Polym. Int. 2017, 66, 1935–1939. [Google Scholar] [CrossRef]
- Hung, W.I.; Lin, Y.H.; Wu, P.S.; Chang, K.C.; Peng, C.W.; Lai, M.C.; Yeh, J.M. Preparation and thermal properties of UV-curable polyacrylate–gold nanocomposite foams. J. Mater. Chem. 2012, 22, 21654–21661. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Real-time kinetic study of laser-induced polymerization. Macromolecules 1989, 22, 4455–4462. [Google Scholar] [CrossRef]
- Avens, H.J.; Bowman, C.N. Mechanism of cyclic dye regeneration during eosinsensitized photoinitiation in the presence of polymerization inhibitors. J. Polym. Sci. Pt. A Polym. Chem. 2009, 47, 6083–6094. [Google Scholar] [CrossRef] [Green Version]
- Decker, C.; Moussa, K. A new method for monitoring ultra-fast photopolymerizations by real-time infra-red (RTIR) spectroscopy. Die Makromol. Chem. Macromol. Chem. Phys. 1988, 189, 2381–2394. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor nanoparticle-based hydrogels prepared via self-initiated polymerization under sunlight, even visible light. Sci. Rep. 2013, 3, 1399. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Ye, J.; Dong, C. Kinetics studies of methyl methacrylate photopolymerization initiated by titanium dioxide semiconductor nanoparticles. J. Photochem. Photobiol. A Chem. 2006, 181, 19–27. [Google Scholar] [CrossRef]
- Sangermano, M.; Marino, F.; Reuel, N.; Strano, M.S. Semiconducting Single-Walled Carbon Nanotubes as Radical Photoinitiators. Macromol. Chem. Phys. 2011, 212, 1469–1473. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Yar, Y.; Acar, H.Y.; Yagci, Y. Magnetic iron oxide nanoparticles as long wavelength photoinitiators for free radical polymerization. Polym. Chem. 2015, 6, 1918–1922. [Google Scholar] [CrossRef] [Green Version]
- Heller, D.A.; Jin, H.; Martinez, B.M.; Patel, D.; Miller, B.M.; Yeung, T.K.; Jena, P.V.; Höbartner, C.; Ha, T.; Silverman, S.K.; et al. Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes. Nat. Nanotechnol. 2009, 4, 114. [Google Scholar] [CrossRef]












| Sample | Tensile Properties | Strain (%) | |
|---|---|---|---|
| Strength (MPa) | Modulus (GPa) | ||
| Neat SLR | 27.8 ± 1.3 | 1.5 ± 0.2 | 4.9 ± 0.5 |
| SLR/Ag-TNP-0.5 | 33.2 ± 0.8 | 1.8 ± 0.1 | 4.5 ± 0.3 |
| SLR/Ag-TNP-0.8 | 39.7 ± 1.1 | 2.0 ± 0.1 | 4.0 ± 0.2 |
| SLR/Ag-TNP-1.0 | 44.7 ± 0.9 | 2.3 ± 0.1 | 3.6 ± 0.4 |
| SLR/Ag-TNP-1.2 | 43.5 ± 0.8 | 2.1 ± 0.1 | 2.9 ± 0.3 |
| Sample | Flexural Properties | Strain (%) | |
|---|---|---|---|
| Strength (MPa) | Modulus (GPa) | ||
| Neat SLR | 42.0 ± 1.2 | 2.2 ± 0.10 | 5.2 ± 0.3 |
| SLR/Ag-TNP-0.5 | 51.5 ± 1.5 | 2.6 ± 0.05 | 4.8 ± 0.2 |
| SLR/Ag-TNP-0.8 | 62.1 ± 1.1 | 2.9 ± 0.10 | 4.3 ± 0.4 |
| SLR/Ag-TNP-1.0 | 70.7 ± 2.1 | 3.5 ± 0.10 | 4.0 ± 0.4 |
| SLR/Ag-TNP-1.2 | 64.7 ± 1.9 | 3.1 ± 0.05 | 3.1 ± 0.5 |
| Sample | Indentation Depth (nm) | Reduced Modulus (GPa) | Hardness (GPa) |
|---|---|---|---|
| Neat SLR | 834.07 | 2.859 ± 0.03 | 0.1662 ± 0.0030 |
| SLR/Ag-TNP-0.5 | 822.16 | 3.118 ± 0.05 | 0.1786 ± 0.0020 |
| SLR/Ag-TNP-0.8 | 774.37 | 3.554 ± 0.04 | 0.1919 ± 0.0020 |
| SLR/Ag-TNP-1.0 | 728.52 | 3.859 ± 0.06 | 0.2251 ± 0.0025 |
| SLR/Ag-TNP-1.2 | 761.06 | 3.559 ± 0.03 | 0.2078 ± 0.0020 |
| Sample | Storage Modulus (MPa) | tan δ Peak Height | Tg (°C) | |
|---|---|---|---|---|
| 30 °C | 100 °C | |||
| Neat SLR | 1495.2 | 245.1 | 0.188 | 79.3 |
| SLR/Ag-TNP-0.5 | 1637.4 | 264.8 | 0.185 | 81.1 |
| SLR/Ag-TNP-0.8 | 1769.9 | 308.9 | 0.182 | 83.2 |
| SLR/Ag-TNP-1.0 | 1953.1 | 350.5 | 0.177 | 86.5 |
| SLR/Ag-TNP-1.2 | 1751.1 | 295.5 | 0.179 | 84.1 |
| Sample | T-50% (°C) | Residual Char (wt%) | Thermal Conductivity (W·m−1·K−1) |
|---|---|---|---|
| Neat SLR | 424.1 | 5.86 | 0.2465 ± 0.003 |
| SLR/Ag-TNP-0.5 | 425.1 | 6.93 | 0.2934 ± 0.005 |
| SLR/Ag-TNP-0.8 | 426.4 | 7.24 | 0.3228 ± 0.004 |
| SLR/Ag-TNP-1.0 | 427.2 | 8.01 | 0.3456 ± 0.005 |
| SLR/Ag-TNP-1.2 | 426.3 | 7.63 | 0.3298 ± 0.004 |
| Nanocomposites | Thermal Conductivity (W·m−1·K−1) | References |
|---|---|---|
| SLR/Ag-TNP-1.0 | 0.34 | This work |
| ® SLR/ANT800 | 0.29 | [74] |
| @ MPTS/hBN-5% | 0.30 | [75] |
| # SU-8/FGS-2% | 0.36 | [76] |
| $ CE/BNNT-1.5% | 0.40 | [77] |
| & UV curable polyacrylate/PGN15 | 0.29 | [78] |
| * UV curable polyacrylate/PGN25 | 0.27 | [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarak, S.; Dhamodharan, D.; B. Kale, M.; Divakaran, N.; Senthil, T.; P., S.; Wu, L.; Wang, J. A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles. Nanomaterials 2020, 10, 217. https://doi.org/10.3390/nano10020217
Mubarak S, Dhamodharan D, B. Kale M, Divakaran N, Senthil T, P. S, Wu L, Wang J. A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles. Nanomaterials. 2020; 10(2):217. https://doi.org/10.3390/nano10020217
Chicago/Turabian StyleMubarak, Suhail, Duraisami Dhamodharan, Manoj B. Kale, Nidhin Divakaran, T. Senthil, Sathiyanathan P., Lixin Wu, and Jianlei Wang. 2020. "A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles" Nanomaterials 10, no. 2: 217. https://doi.org/10.3390/nano10020217
APA StyleMubarak, S., Dhamodharan, D., B. Kale, M., Divakaran, N., Senthil, T., P., S., Wu, L., & Wang, J. (2020). A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles. Nanomaterials, 10(2), 217. https://doi.org/10.3390/nano10020217