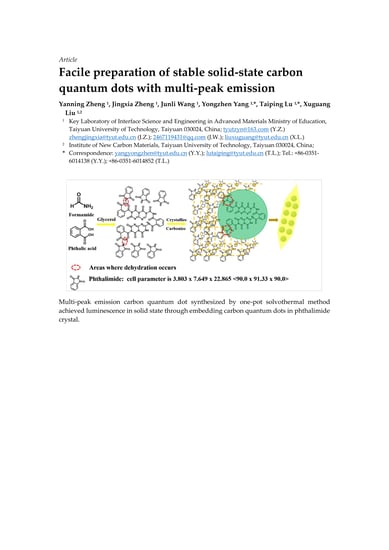
Facile Preparation of Stable Solid-State Carbon Quantum Dots with Multi-Peak Emission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of CQDs/PC
2.3. Fabrication of WLED
2.4. Characterization
3. Results and Discussion
3.1. Morphology and Structures
3.2. Optical Properties and Thermal Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, Y.B.; Zhu, S.J.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Bai, X.; Zhai, Y.; Chen, X.; Zhu, Y.S.; Pan, G.C.; Zhang, H.Z.; Dong, B.; Song, H.W. Carbon dots with efficient solid-state photoluminescence towards white light-emitting diodes. J. Mater. Chem. C 2017, 5, 11416–11420. [Google Scholar] [CrossRef]
- Xie, Y.T.; Zheng, J.X.; Wang, Y.L.; Wang, J.L.; Yang, Y.Z.; Liu, X.G.; Chen, Y.K. One-step hydrothermal synthesis of fluorescence carbon quantum dots with high product yield and quantum yield. Nanotechnology 2019, 30, 085406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.L.; Yuan, T.; Sui, L.Z.; Wang, Z.B.; Xi, Z.F.; Li, Y.C.; Li, X.H.; Fan, L.Z.; Tan, Z.A.; Chen, A.M.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Wang, Z.B.; Li, X.H.; Li, Y.C.; Tan, Z.A.; Fan, L.Z.; Yang, S.H. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mate. 2017, 29, 1604436. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, S.Y.; Sun, X.M.; Li, X.M.; Zeng, H.B.; Xie, Z.; Chen, P.; Zhou, S.Y. Highly efficient carbon dots with reversibly switchable green–red emissions for trichromatic white light-emitting diodes. ACS Appl. Mater. Inter. 2018, 10, 16005–16014. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, T.; Xu, M.; Zhang, M.R.; Guo, Y.J.; Zhang, L.P.; Street, J.; Ong, W.J.; Xu, Q. Full color carbon dots through surface engineering for constructing white light-emitting diodes. J. Mater. Chem. C 2019, 7, 2212–2218. [Google Scholar] [CrossRef]
- Yuan, B.; Xie, Z.; Chen, P.; Zhou, S.Y. Highly efficient carbon dots and their nanohybrids for trichromatic white LEDs. J. Mater. Chem. C 2018, 6, 5957–5963. [Google Scholar] [CrossRef]
- Chen, S.M.; Yan, C.M.; Tang, Y.; Li, J.S.; Ding, X.R.; Rao, L.S.; Li, Z.T. Improvement in luminous efficacy and thermal performance using quantum dots spherical shell for white light emitting diodes. Nanomaterials 2018, 8, 618. [Google Scholar] [CrossRef] [Green Version]
- George, N.C.; Denault, K.A.; Seshadri, R. Phosphors for solid-state white lighting. Annu. Rev. Mater. Res. 2013, 43, 481–501. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-emissive carbon dots for fingerprints detection by spray method: Coffee ring effect and unquenched fluorescence in drying process. ACS Appl. Mater. Inter. 2017, 9, 18429–18433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Yuan, F.L.; Li, X.H.; Li, Y.C.; Zhong, H.Z.; Fan, L.Z.; Yang, S.H. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.G.; Cai, C.Z.; Lin, H.W. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.N.; Zhou, D.; Li, D.; Ji, W.Y.; Jing, P.T.; Han, D.; Liu, L.; Zeng, H.B.; Shen, D.Z. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.Y.; Wang, Y.H. Highly green emissive nitrogen-doped carbon dots with excellent thermal stability for bioimaging and solid-state LED. Inorg. Chem. 2018, 57, 15229–15239. [Google Scholar] [CrossRef]
- Zheng, J.X.; Wang, Y.L.; Zhang, F.; Yang, Y.Z.; Liu, X.G.; Guo, K.P.; Wang, H.; Xu, B.S. Microwave-assisted hydrothermal synthesis of solid-state carbon dots with intensive emission for white light-emitting devices. J. Mater. Chem. C 2017, 5, 8105–8111. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zheng, M.T.; Xiao, Y.; Dong, H.W.; Zhang, H.R.; Zhuang, J.L.; Hu, H.; Lei, B.F.; Liu, Y.L. A self-quenching-resistant carbon-dot powder with tunable solid-state fluorescence and construction of dual-fluorescence morphologies for white light-emission. Adv. Mater. 2016, 28, 312–318. [Google Scholar] [CrossRef]
- Sun, M.Y.; Qu, S.N.; Hao, Z.D.; Ji, W.Y.; Jing, P.T.; Zhang, H.; Zhang, L.G.; Zhao, J.L.; Shen, D.Z. Towards efficient solid-state photoluminescence based on carbon-nanodots and starch composites. Nanoscale 2014, 6, 13076–13081. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.C.; Wang, Y.; Li, D.; Zhou, D.; Jing, P.T.; Shen, D.Z.; Qu, S.N. Red carbon dots-based phosphors for white light-emitting diodes with color rendering index of 92. J. Colloid Interf. Sci. 2018, 528, 281–288. [Google Scholar] [CrossRef]
- Chen, D.Q.; Chen, X.; Gao, H.B.; Zhong, J.S. Red C-dots and C-dot films: Solvothermal synthesis, excitation-independent emission and solid-state-lighting. RSC Adv. 2018, 8, 29855–29861. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Li, D.; Jing, P.T.; Zhai, Y.C.; Shen, D.Z.; Qu, S.N.; Rogach, A.L. Conquering aggregation-induced solid-state luminescence quenching of carbon dots through a carbon dots-triggered silica gelation process. Chem. Mater. 2017, 29, 1779–1787. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.D.; Lulu, L.; Song, Q.J. Rapid visualization of latent fingerprints with color-tunable solid fluorescent carbon dots. Part. Part. Syst. Char. 2018, 35, 1700387. [Google Scholar] [CrossRef]
- Chang, Q.; Ding, Y.M.; Cheng, S.; Shen, W.; Zhou, Z.; Yin, Y.H.; Sun, T.; Ban, C.Y.; Deng, Z.T.; Liu, J.Q.; et al. Quench-resistant and stable nanocarbon dot/sheet emitters with tunable solid-state fluorescence via aggregation-induced color switching. Nanoscale 2019, 11, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.S.; Anthony, B.P.; Dirk, P. Selecting conversion phosphors for white light-emitting diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar]
- Wang, X.F.; Wang, G.G.; Li, J.B.; Liu, Z.; Zhao, W.F.; Han, J.C. Towards high-powered remote WLED based on flexible white-luminescent polymer composite films containing S, N co-doped graphene quantum dots. Chem. Eng. J. 2018, 336, 406–415. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.X.; Nie, B.; Zhao, Y.K.; Fan, H.Y.; Sun, Z.C. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.T.; Chen, Y.Y.; Xu, Y.L.; Song, Q.J. Polymer assisted self-assembly of multicolor carbon-dots as solid-state phosphors for fabrication of warm, high-quality and temperature responsive white light-emitting devices. ACS Appl. Mater. Inter. 2019, 11, 22332–22338. [Google Scholar] [CrossRef]
- Zhao, F.F.; Zhang, T.Y.; Liu, Q.; Lü, C.L. Aphen-derived N-doped white-emitting carbon dots with room temperature phosphorescence for versatile applications. Sensor. Actuat. B-Chem. 2020, 304, 127344. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhao, F.F.; Li, L.; Qi, B.; Zhu, D.X.; Lü, J.H.; Lü, C.L. Tricolor white-light-emitting carbon dots with multiple-cores @ shell structure for WLED application. ACS Appl. Mater. Inter. 2018, 10, 19796–19805. [Google Scholar] [CrossRef]
- Qu, S.N.; Shen, D.Z.; Liu, X.Y.; Jing, P.T.; Zhang, L.G.; Ji, W.Y.; Zhao, H.F.; Fan, X.W.; Zhang, H. Highly luminescent carbon-nanoparticle-based materials: Factors influencing photoluminescence quantum yield. Part. Part. Sys. Char. 2014, 31, 1175–1182. [Google Scholar] [CrossRef]
- Yan, F.Y.; Bai, Z.J.; Zu, F.L.; Zhang, Y.; Sun, X.D.; Ma, T.C.; Chen, L. Yellow-emissive carbon dots with a large Stokes shift are viable fluorescent probes for detection and cellular imaging of silver ions and glutathione. Microchim. Acta. 2019, 186, 133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhou, Q.X.; Yuan, Y.Y.; Wu, Y.L. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.Y.; Wang, B.; Li, H.Y.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.J.; Wang, H.Y.; Qu, S.N.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Xu, H.L.; Han, W.; Yang, B.; et al. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Lian, H.Z.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.G.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef]
- Pan, L.L.; Sun, S.; Zhang, A.D.; Jiang, K.; Zhang, L.; Dong, C.Q.; Huang, Q.; Wu, A.G.; Lin, H.W. Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv. Mater. 2015, 27, 7782–7787. [Google Scholar] [CrossRef]
- Dang, H.; Huang, L.K.; Zhang, Y.; Wang, C.F.; Chen, S. Large-scale ultrasonic fabrication of white fluorescent carbon dots. Ind. Eng. Chem. Res. 2016, 55, 5335–5341. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zheng, J.; Wang, J.; Yang, Y.; Lu, T.; Liu, X. Facile Preparation of Stable Solid-State Carbon Quantum Dots with Multi-Peak Emission. Nanomaterials 2020, 10, 303. https://doi.org/10.3390/nano10020303
Zheng Y, Zheng J, Wang J, Yang Y, Lu T, Liu X. Facile Preparation of Stable Solid-State Carbon Quantum Dots with Multi-Peak Emission. Nanomaterials. 2020; 10(2):303. https://doi.org/10.3390/nano10020303
Chicago/Turabian StyleZheng, Yanning, Jingxia Zheng, Junli Wang, Yongzhen Yang, Taiping Lu, and Xuguang Liu. 2020. "Facile Preparation of Stable Solid-State Carbon Quantum Dots with Multi-Peak Emission" Nanomaterials 10, no. 2: 303. https://doi.org/10.3390/nano10020303
APA StyleZheng, Y., Zheng, J., Wang, J., Yang, Y., Lu, T., & Liu, X. (2020). Facile Preparation of Stable Solid-State Carbon Quantum Dots with Multi-Peak Emission. Nanomaterials, 10(2), 303. https://doi.org/10.3390/nano10020303