Graphene-Ionic Liquid Thin Film Nanolubricant
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Rheological Behavior
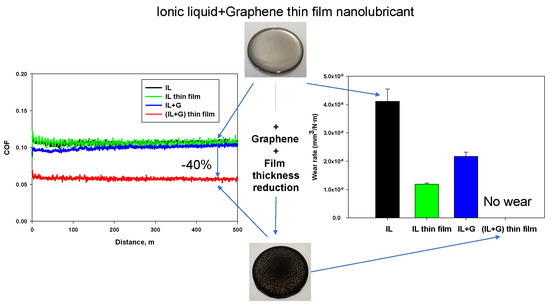
3.2. Friction Coefficients and Wear Rates
3.3. Surface Analysis and Wear Mechanism
4. Conclusions
- (1)
- A new dispersion of graphene in the hydrophobic ionic liquid 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl) imide was obtained and its non-Newtonian behavior was characterized. The viscosity of the new dispersion decreases with increasing temperatures, but it is maintained from 100 to 150 °C.
- (2)
- The tribological performance of the neat ionic liquid and of the dispersion of graphene in ionic liquid was compared with that of thin films of the same lubricants deposited by spin coating on the stainless steel surface. All lubricants present constant friction coefficients along the tribological tests.
- (3)
- The best friction-reducing ability (40% with respect to the rest of lubricants) is obtained for the new spin-coated graphene dispersion in ionic liquid, by the combination of addition of graphene and film thickness reduction.
- (4)
- Wear rate of stainless steel is reduced up to 70% by ionic liquid film thickness reduction, and up to 54% by dispersion of graphene in thick film lubrication.
- (5)
- Surface damage and materials loss are only completely prevented by the combination of both factors, addition of graphene and thickness reduction, in the thin film spin coated dispersion of graphene in ionic liquid. This outstanding anti-wear performance is attributed to the sliding of ionic liquid modified graphene sheets, and to the absence of tribocorrosion at the interface.
Author Contributions
Funding
Conflicts of Interest
References
- Ye, C.F.; Liu, W.M.; Chen, Y.X.; Yu, L.G. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.D.; Jimenez, A.E.; Sanes, J.; Carrion, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Chen, H.; Shi, X.N.; Liu, X.; Duan, G.J. Tribological properties of ionic liquids for Steel/aluminum, Steel-copper and Steel/Si3N4 ceramic contact under boundary lubrication. Ind. Lubr. Tribol. 2018, 70, 1158–1168. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Bermudez, M.D.; Iglesias, P.; Carrion, F.J.; Martinez-Nicolas, G. 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel-aluminium contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Sanes, J.; Carrion, F.J.; Bermudez, M.D.; Martinez-Nicolas, G. Ionic liquids as lubricants of polystyrene and polyamide 6-steel contacts. Preparation and properties of new polymer-ionic liquid dispersions. Tribol. Lett. 2006, 21, 121–133. [Google Scholar] [CrossRef]
- Zheng, D.; Cai, Z.B.; Shen, M.X.; Li, Z.Y.; Zhu, M.H. Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron surface. Appl. Surf. Sci. 2016, 387, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Liñeira del Rio, J.M.; Guimarey, M.J.G.; Comunas, M.J.P.; Lopez, E.R.; Amigo, A.; Fernandez, J. Thermophysical and tribological properties of dispersions based on graphene and a trimethylolpropane trioleate oil. J. Mol. Liquids 2018, 268, 854–866. [Google Scholar] [CrossRef]
- Sun, J.L.; Du, S.N. Application of graphene derivatives and their nanocomposites in tribology and lubrication: A review. RSC Adv. 2019, 9, 40642–40661. [Google Scholar] [CrossRef] [Green Version]
- Aviles, M.D.; Saurin, N.; Sanes, J.; Carrion, F.J.; Bermudez, M.D. Ionanocarbon lubricants. The combination of ionic liquids and carbon nanophases in tribology. Lubricants 2017, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.; Aviles, M.D.; Saurin, N.; Espinosa, T.; Carrion, F.J.; Bermudez, M.D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Saurin, N.; Aviles, M.D.; Espinosa, T.; Sanes, J.; Carrion, F.J.; Bermudez, M.D.; Iglesias, P. Carbon nanophases in ordered nanofluid lubricants. Wear 2017, 376, 747–755. [Google Scholar] [CrossRef]
- Saurin, N.; Sanes, J.; Bermudez, M.D. New graphene/ionic liquid nanolubricants. Mater. Today Proc. 2016, 3, S227–S232. [Google Scholar] [CrossRef]
- Senatore, A.; Pisaturo, M.; Guida, D. Polyalkylene glycol based lubricants and tribological behaviour: Role of ionic liquids and graphene oxide as additives. J. Nanosci. Nanotechnol. 2018, 18, 913–924. [Google Scholar] [CrossRef]
- Zhang, L.L.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Liu, X.F.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Novel DLC/ionic liquid/graphene nanocomposite coatings towards high-vacuum related space applications. J. Mater. Chem. A 2013, 1, 3797–3809. [Google Scholar] [CrossRef]
- Pu, J.B.; Wan, S.H.; Zhao, W.J.; Mo, Y.F.; Zhang, X.Q.; Wang, L.P.; Xue, Q.J. Preparation and tribological study of functionalized graphene-IL nanocomposite ultrathin lubrication films on Si substrates. J. Phys. Chem. C 2011, 115, 13275–13284. [Google Scholar] [CrossRef]
- Khare, V.; Pham, M.Q.; Kumari, N.; Yoon, H.S.; Kim, C.S.; Park, J.I.; Ahn, S.H. Graphene-ionic liquid based hybrid nanomaterials as novel lubricant for low friction and wear. ACS Appl. Mater. Interfaces 2013, 5, 4063–4075. [Google Scholar] [CrossRef]
- Fan, X.Q.; Wang, L.P.; Li, W. In situ fabrication of low-friction sandwich sheets through functionalized graphene crosslinked by ionic liquids. Tribol. Lett. 2015, 58, 12. [Google Scholar] [CrossRef]
- Fan, X.Q.; Wang, L.P. High-performance lubricant additives based on modified graphene oxide by ionic liquids. J. Coll. Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Mungse, H.P.; Kumar, N.; Ravindran, T.R.; Pandian, R.; Sugimura, H.; Khatri, O.P. Covalently attached graphene-ionic liquid hybrid nanomaterials: Synthesis, characterization and tribological application. J. Mater. Chem. A 2016, 4, 926–937. [Google Scholar] [CrossRef]
- Zhao, W.J.; Zeng, Z.X.; Peng, S.S.; Wu, X.D.; Xue, Q.J.; Chen, J.M. Fabrication and investigation the microtribological behaviors of ionic liquid-graphene composite films. Tribol. Trans. 2013, 56, 480–487. [Google Scholar] [CrossRef]
- Fu, H.M.; Fan, X.Q.; Li, W.; Zhu, M.H.; Peng, J.F.; Li, H. In situ modified multilayer graphene toward high-performance lubricating additive. RSC Adv. 2017, 7, 24399–24409. [Google Scholar] [CrossRef] [Green Version]
- Avilés, M.D.; Jiménez, A.E.; Saurín, N.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Tribological characterization of epoxy coatings modified with ionic liquids and graphene. Tribol. Int. 2020, 105516. [Google Scholar] [CrossRef]
- Carstens, T.; Gustus, R.; Höfft, O.; Borisenko, N.; Endres, F.; Li, H.; Wood, R.J.; Page, A.J. Combined STM, AFM, and DFT study of the highly ordered pyrolytic graphite/1-octyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide interface. J. Phys. Chem. C 2014, 118, 10833–10843. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.H.; Eren, B.; Hao, Y.; Feng, B.; Fang, H.T.; Salmerón, M. Operando STM study of the interaction of imidazolium-based ionic liquid with graphite. Energy Storage Mater. 2019, 20, 139–145. [Google Scholar] [CrossRef] [Green Version]
- He, T.X.; Dai, Q.W.; Huang, W.; Wang, X.L. Colloidal suspensions of Graphene oxide in ionic liquid as lubricant. Appl. Phys. A Mater. Sci. Process. 2018, 124, 777. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Branco, L.C.; Crespo, J.G.; Nunes, M.C.; Raymundo, A.; Afonso, C.A.M. Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium and guanidinium cations. Chem. Eur. J. 2007, 13, 8478–8488. [Google Scholar] [CrossRef] [PubMed]
- Pamies, R.; Avilés, M.D.; Arias-Pardilla, J.; Espinosa, T.; Carrión, F.J.; Sanes, J.; Bermudez, M.D. Antiwear performance of ionic liquid plus graphene dispersions with anomalous viscosity-temperature behavior. Tribol. Int. 2018, 122, 200–209. [Google Scholar] [CrossRef]
- Avilés, M.D.; Carrión-Vilches, F.J.; Sanes, J.; Bermúdez, M.D. Diprotic ammonium succinate ionic liquid in thin film aqueous lubrication and in graphene nanolubricant. Tribol. Lett. 2019, 67, 26. [Google Scholar] [CrossRef]
- Cremer, T. Ionic Liquid Bulk and Interface Properties; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Xu, C.Y.; Zhang, P.X.; Yan, L. Blue shift of Raman peak from coated TiO2 nanoparticles. J. Raman Spectrosc. 2001, 32, 862–865. [Google Scholar] [CrossRef]
- Ni, Z.; Wuan, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef] [Green Version]















| Parameter | Value |
|---|---|
| AISI 316L disk thickness (mm) | 2.5 |
| AISI 316L disk diameter (mm) | 25 |
| AISI 316L surface roughness (Ra; μm) | <0.15 |
| Normal load (N) | 0.5 |
| Maximum contact pressure (GPa) | 1.56 |
| Mean contact pressure (GPa) | 1.04 |
| Sliding speed (m·s−1) | 0.01 |
| Sliding distance (m) | 500 |
| Sliding radius (mm) | 9 |
| Sapphire ball sphere radius (mm) | 0.75 |
| Lubricant volume (mL) | 0.2 |
| Temperature (°C) | 23 ± 1 |
| Relative humidity (%) | 55 ± 5 |
| Lubricant | Coefficient of Friction | Wear Rate (mm3/N·m) |
|---|---|---|
| IL | 0.10 (±0.009) | 4.1 × 10−6 (±4.37 × 10−7) |
| IL + G | 0.10 (±0.009) | 2.2 × 10−6 (±1.53 × 10−7) |
| IL thin film | 0.10 (±0.009) | 1.2 × 10−6 (±4.48 × 10−8) |
| (IL + G) thin film | 0.06 (±0.006) | Non measurable |
| Lubricant | Ra (Inside the Sliding Paths; μm) | Ra (Outside the Sliding Paths; μm) |
|---|---|---|
| IL | 1.47 × 10−1 (±1.6 × 10−2) | 5.79 × 10−2 (±5.2 × 10−3) |
| IL + G | 1.29 × 10−1 (±1.6 × 10−2) | 5.50 × 10−2 (±4.8 × 10−3) |
| IL thin film | 8.79 × 10−2 (±4.7 × 10−3) | 4.88 × 10−2 (±1.3 × 10−3) |
| (IL + G) thin film | 7.75 × 10−2 (±9.5 × 10−3) | 5.33 × 10−2 (±4.4 × 10−3) |
| Element | Outside | Inside | ||
|---|---|---|---|---|
| Binding Energy (eV) | Atomic % | Binding Energy (eV) | Atomic % | |
| C1s | 285.0 | 27.9 | 285.0 | 37.9 |
| 286.5 | 4.5 | 286.9 | 4.2 | |
| 288.7 | 6.4 | 288.8 | 5.1 | |
| 293.0 | 0.7 | 293.2 | 1.0 | |
| O1s | 530.0 | 20.1 | 530.1 | 17.4 |
| 531.7 | 25.6 | 531.5 | 10.3 | |
| 532.3 | 12.5 | |||
| 533.1 | 2.1 | |||
| N1s | 398.2 | 0.3 | 398.9 | 0.5 |
| 400.0 | 0.8 | 400.3 | 0.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés, M.-D.; Pamies, R.; Sanes, J.; Bermúdez, M.-D. Graphene-Ionic Liquid Thin Film Nanolubricant. Nanomaterials 2020, 10, 535. https://doi.org/10.3390/nano10030535
Avilés M-D, Pamies R, Sanes J, Bermúdez M-D. Graphene-Ionic Liquid Thin Film Nanolubricant. Nanomaterials. 2020; 10(3):535. https://doi.org/10.3390/nano10030535
Chicago/Turabian StyleAvilés, María-Dolores, Ramón Pamies, José Sanes, and María-Dolores Bermúdez. 2020. "Graphene-Ionic Liquid Thin Film Nanolubricant" Nanomaterials 10, no. 3: 535. https://doi.org/10.3390/nano10030535
APA StyleAvilés, M. -D., Pamies, R., Sanes, J., & Bermúdez, M. -D. (2020). Graphene-Ionic Liquid Thin Film Nanolubricant. Nanomaterials, 10(3), 535. https://doi.org/10.3390/nano10030535