Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Reduced Graphene Oxide (MRGO)
2.3. Characterization
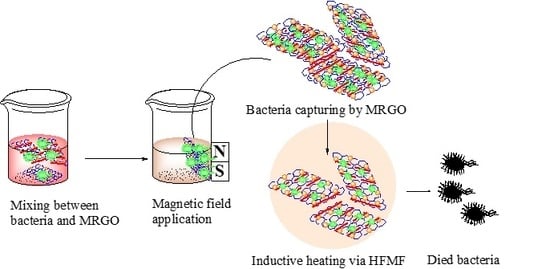
2.4. Bacterial Culture Assessment and Bacteria Captured by MRGO
2.5. Magnetic Inductive Heating (Hyperthermia) Experiment by HFMF
3. Results and Discussion
3.1. Physicochemical Characterizations
3.2. Capturing Capability of Bacteria
3.3. Hyperthermia Experiment and Bacteria Capturing and Removing Activity Assisted by HFMF Exposure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.; Wang, Y.; Chen, S.; Han, N.; Liu, H.; Zhang, X. Multiresponsive Shape-Stabilized Hexadecyl Acrylate-Grafted Graphene as a Phase Change Material with Enhanced Thermal and Electrical Conductivities. ACS Appl. Mater. Interfaces 2019, 11, 8982–8991. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N.; Wu, H.-F. Multifunctional graphene magnetic nanosheet decorated with chitosan for highly sensitive detection of pathogenic bacteria. J. Mater. Chem. B 2013, 1, 3950–3961. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, Facts, Opportunities, and Challenges in Nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Singh, R.; Sreedharan, S.; Karuppiah, S.; Singh, S. Enhanced bioactivity of GO–Fe3O4 nanocomposite against pathogenic bacterial strains. Int. J. Nanomed. 2018, 13, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Yan, L.; Chang, Y.-N.; Zhao, L.; Gu, Z.; Liu, X.; Tian, G.; Zhou, L.; Ren, W.; Jin, S.; Yin, W.; et al. The use of polyethylenimine-modified graphene oxide as a nanocarrier for transferring hydrophobic nanocrystals into water to produce water-dispersible hybrids for use in drug delivery. Carbon 2013, 57, 120–129. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Moreira, S.; Goncalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhaes, F.D. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xiao, Y.; Bergeret, A.; Longerey, M.; Che, J. Preparation of polylactide/graphene composites from liquid-phase exfoliated graphite nanosheets. Polym. Compos. 2014, 35, 396–403. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 2010, 46, 2622–2626. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Zhu, H.; Yang, X. Synthesis of graphene nanosheets with incorporated silver nanoparticles for enzymeless hydrogen peroxide detection. Anal. Methods 2013, 5, 2298–2304. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Liu, X.-L.; Herng, T.S.; Peng, E.; Ding, J. GO-Functionalized Large Magnetic Iron Oxide Nanoparticles with Enhanced Colloidal Stability and Hyperthermia Performance. ACS Appl. Mater. Interfaces 2019, 11, 22703–22713. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of Iron Oxide-Based Magnetic Nanoparticles in the Diagnosis and Treatment of Bacterial Infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Diniz da Costa, J.C. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yong, C.; Cao, W.; Wang, X.; Wang, L.; Zhou, J.; Xing, X. Fabrication of charge reversible graphene oxide-based nanocomposite with multiple antibacterial modes and magnetic recyclability. J. Colloid Interface Sci. 2018, 511, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, M.; Li, N.; Yan, B.; Ma, H.; Hu, Y.; Ye, M.J.N.R. Facile synthesis and application of Ag-chemically converted graphene nanocomposite. Nano Res. 2010, 3, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Hu, B.; Wang, N.; Han, L.; Chen, M.-L.; Wang, J.-H. Magnetic Nanohybrids Loaded with Bimetal Core–Shell–Shell Nanorods for Bacteria Capture, Separation, and Near-Infrared Photothermal Treatment. Chem. A Eur. J. 2015, 21, 6582–6589. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Vasyukova, I.; Muratov, D.S.; Rybkin, I.; Bratashov, D.; Lapanje, A.; Il’inikh, I.; Kolesnikov, E.; Kuznetsov, D. Effect of GO on bacterial cells: Role of the medium type and electrostatic interactions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 275–281. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Shaiu, R.S.; Liu, D.M.; Chen, S.Y. Instantaneous drug delivery of magnetic/thermally sensitive nanospheres by a high-frequency magnetic field. Langmuir 2008, 24, 13306–13311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-Y.; Liu, T.-Y.; Chan, T.-Y.; Tsai, S.-C.; Hardiansyah, A.; Huang, L.-Y.; Yang, M.-C.; Lu, R.-H.; Jiang, J.-K.; Yang, C.-Y.; et al. Magnetically triggered nanovehicles for controlled drug release as a colorectal cancer therapy. Colloids Surf. B Biointerfaces 2016, 140, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.-Z.; Zhao, D.-L.; Xu, Y.; Zhang, J.-M.; Gao, Y.-L.; Zhao, L.-Y.; Tang, J.-T. Inductive heating property of graphene oxide–Fe3O4 nanoparticles hybrid in an AC magnetic field for localized hyperthermia. Mater. Lett. 2012, 68, 399–401. [Google Scholar] [CrossRef]
- Szabó, T.; Nánai, L.; Nesztor, D.; Barna, B.; Malina, O.; Tombácz, E. A Simple and Scalable Method for the Preparation of Magnetite/Graphene Oxide Nanocomposites under Mild Conditions. Adv. Mater. Sci. Eng. 2018, 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Mevold, A.H.; Hsu, W.W.; Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Chan, T.Y.; Wang, K.S.; Su, Y.A.; Jeng, R.J.; et al. Fabrication of Gold Nanoparticles/Graphene-PDDA Nanohybrids for Bio-detection by SERS Nanotechnology. Nanoscale Res. Lett. 2015, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Wang, S.; Wang, X.; Shen, G.; Lin, J.; Wang, Z.; Guo, S.; Cui, D.; Yang, M.; Chen, X. Surface Functionalization of Chemically Reduced Graphene Oxide for Targeted Photodynamic Therapy. J. Biomed. Nanotechnol. 2015, 11, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Huang, L.-Y.; Hu, S.-H.; Yang, M.-C.; Chen, S.-Y. Core–Shell Magnetic Nanoparticles of Heparin Conjugate as Recycling Anticoagulants. J. Biomed. Nanotechnol. 2007, 3, 353–359. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L.; Chang, D.W.; Baek, J.B. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Zhu, D.; Ma, S.; Yu, W.; Jia, Y.; Li, Y.; Yu, H.; Shen, Z. Highly Efficient Removal of Pathogenic Bacteria with Magnetic Graphene Composite. ACS Appl. Mater. Interfaces 2015, 7, 4290–4298. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Chen, C.-L.; Lee, Y.-C.; Chan, T.-Y.; Wang, Y.-L.; Lin, J.-J. First Observation of Physically Capturing and Maneuvering Bacteria using Magnetic Clays. ACS Appl. Mater. Interfaces 2016, 8, 411–418. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardiansyah, A.; Yang, M.-C.; Liao, H.-L.; Cheng, Y.-W.; Destyorini, F.; Irmawati, Y.; Liu, C.-M.; Yung, M.-C.; Hsu, C.-C.; Liu, T.-Y. Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field. Nanomaterials 2020, 10, 674. https://doi.org/10.3390/nano10040674
Hardiansyah A, Yang M-C, Liao H-L, Cheng Y-W, Destyorini F, Irmawati Y, Liu C-M, Yung M-C, Hsu C-C, Liu T-Y. Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field. Nanomaterials. 2020; 10(4):674. https://doi.org/10.3390/nano10040674
Chicago/Turabian StyleHardiansyah, Andri, Ming-Chien Yang, Hung-Liang Liao, Yu-Wei Cheng, Fredina Destyorini, Yuyun Irmawati, Chi-Ming Liu, Ming-Chi Yung, Chuan-Chih Hsu, and Ting-Yu Liu. 2020. "Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field" Nanomaterials 10, no. 4: 674. https://doi.org/10.3390/nano10040674
APA StyleHardiansyah, A., Yang, M. -C., Liao, H. -L., Cheng, Y. -W., Destyorini, F., Irmawati, Y., Liu, C. -M., Yung, M. -C., Hsu, C. -C., & Liu, T. -Y. (2020). Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field. Nanomaterials, 10(4), 674. https://doi.org/10.3390/nano10040674