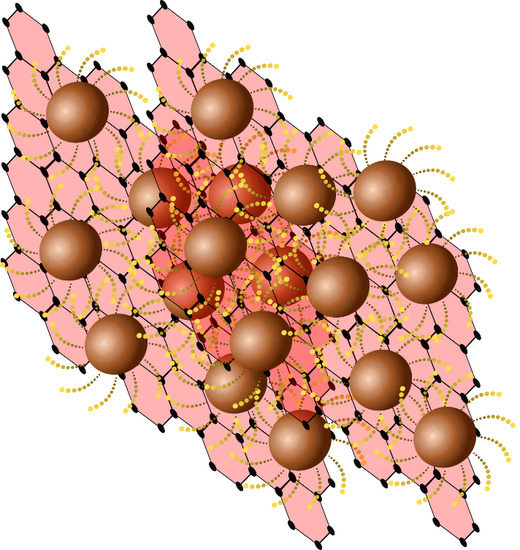
Ligand-Assisted Formation of Graphene/Quantum Dot Monolayers with Improved Morphological and Electrical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. rGO Functionalization
2.3. PbS Linking
2.4. Solution-Phase Ligand Exchange
2.5. LB Film Deposition
2.6. Post-Deposition Ligand Exchange
2.7. Microscopy
2.8. Spectroscopy
2.9. J-V Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Y.; Zhang, W.; Chi, H.; Liu, Y.; Hou, C.L.; Fang, D. Recent development of graphene materials applied in polymer solar cell. Renew. Sustain. Energy Rev. 2015, 43, 973–980. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, J.; He, Q.; Cao, X.; Tan, C.; Chen, H.; Yan, Q.; Zhang, H. Graphene-Based materials for solar cell applications. Adv. Energy Mater. 2014, 4, 1300574. [Google Scholar] [CrossRef]
- Lin, X.F.; Zhang, Z.Y.; Yuan, Z.K.; Li, J.; Xiao, X.F.; Hong, W.; Chen, X.D.; Yu, D.S. Graphene-based materials for polymer solar cells. Chin. Chem. Lett. 2016, 27, 1259–1270. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.B. Graphene and its derivatives for solar cells application. Nano Energy 2018, 47, 51–65. [Google Scholar] [CrossRef]
- Litvin, A.P.; Martynenko, I.V.; Purcell-Milton, F.; Baranov, A.V.; Fedorov, A.V.; Gun’Ko, Y.K. Colloidal quantum dots for optoelectronics. J. Mater. Chem. A 2017, 5, 13252–13275. [Google Scholar] [CrossRef]
- Litvin, A.P.; Zhang, X.; Berwick, K.; Fedorov, A.V.; Zheng, W.; Baranov, A.V. Carbon-based interlayers in perovskite solar cells. Renew. Sustain. Energy Rev. 2020, 124, 109774. [Google Scholar] [CrossRef]
- Yun, J.M.; Yeo, J.S.; Kim, J.; Jeong, H.G.; Kim, D.Y.; Noh, Y.J.; Kim, S.S.; Ku, B.C.; Na, S.I. Solution-processable reduced graphene oxide as a novel alternative to PEDOT:PSS hole transport layers for highly efficient and stable Polymer solar cells. Adv. Mater. 2011, 23, 4923–4928. [Google Scholar] [CrossRef]
- Hou, Y.X.; Geng, X.M.; Li, Y.Z.; Dong, B.; Liu, L.W.; Sun, M.T. Electrical and Raman properties of p-type and n-type modified graphene by inorganic quantum dot and organic molecule modification. Sci. China Phys. Mech. Astron. 2011, 54, 416–419. [Google Scholar] [CrossRef]
- Wang, R.; Shang, Y.; Kanjanaboos, P.; Zhou, W.; Ning, Z.; Sargent, E.H. Colloidal quantum dot ligand engineering for high performance solar cells. Energy Environ. Sci. 2016, 9, 1130–1143. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, J.; Hawks, S.A.; Shi, G.; Lee, S.-T.; Ma, W. Photovoltaic Devices Based on Colloidal PbX Quantum Dots: Progress and Prospects. Sol. RRL 2017, 1, 1600021. [Google Scholar] [CrossRef]
- Shrestha, A.; Batmunkh, M.; Tricoli, A.; Qiao, S.Z.; Dai, S. Near-Infrared Active Lead Chalcogenide Quantum Dots: Preparation, Post-Synthesis Ligand Exchange, and Applications in Solar Cells. Angew. Chem.-Int. Ed. 2019, 58, 5202–5224. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Neo, D.C.J.; Hou, B.; Park, J.B.; Cho, Y.; Zhang, N.; Hong, J.; Pak, S.; Lee, S.; Sohn, J.I.; et al. High Performance PbS Quantum Dot/Graphene Hybrid Solar Cell with Efficient Charge Extraction. ACS Appl. Mater. Interfaces 2016, 8, 13902–13908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Li, D.B.; Gao, L.; Tan, H.; Chen, C.; Li, K.; Li, M.; Han, J.B.; Song, H.; Liu, H.; et al. Graphene Doping Improved Device Performance of ZnMgO/PbS Colloidal Quantum Dot Photovoltaics. Adv. Funct. Mater. 2016, 26, 1899–1907. [Google Scholar] [CrossRef]
- Martín-García, B.; Bi, Y.; Prato, M.; Spirito, D.; Krahne, R.; Konstantatos, G.; Moreels, I. Reduction of moisture sensitivity of PbS quantum dot solar cells by incorporation of reduced graphene oxide. Sol. Energy Mater. Sol. Cells 2018, 183, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mnoyan, A.; Kim, K.; Kim, J.Y.; Jeon, D.Y. Colloidal nanocomposite of reduced graphene oxide and quantum dots for enhanced surface passivation in optoelectronic applications. Sol. Energy Mater. Sol. Cells 2016, 144, 181–186. [Google Scholar] [CrossRef]
- Konstantatos, G.; Badioli, M.; Gaudreau, L.; Osmond, J.; Bernechea, M.; De Arquer, F.P.G.; Gatti, F.; Koppens, F.H.L. Hybrid grapheneĝquantum dot phototransistors with ultrahigh gain. Nat. Nanotechnol. 2012, 7, 363–368. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Li, J.; Tai, G.A.; Lau, S.P.; Yan, F. Infrared photodetectors based on CVD-grown graphene and PbS quantum dots with ultrahigh responsivity. Adv. Mater. 2012, 24, 5878–5883. [Google Scholar] [CrossRef]
- Turyanska, L.; Makarovsky, O.; Svatek, S.A.; Beton, P.H.; Mellor, C.J.; Patanè, A.; Eaves, L.; Thomas, N.R.; Fay, M.W.; Marsden, A.J.; et al. Ligand-Induced Control of Photoconductive Gain and Doping in a Hybrid Graphene–Quantum Dot Transistor. Adv. Electron. Mater. 2015, 1, 1500062. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.T.; Wang, H.Y.; Song, X.X.; Jin, L.F.; Yao, J.Q. Wide spectral response field-effect phototransistor based on graphene-quantum dot hybrid. IEEE Photonics J. 2015, 7, 4500706. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Zhu, R.J.; Kang, N.; Du, J.; Xu, H.Q. Photoelectrical response of hybrid graphene-PbS quantum dot devices. Appl. Phys. Lett. 2013, 103, 143119. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Rios, J.M.; Sukharevska, N.; Speirs, M.J.; Jung, S.; Dirin, D.; Dragoman, R.M.; Allard, S.; Kovalenko, M.V.; Scherf, U.; Loi, M.A. Enhancing Quantum Dot Solar Cells Stability with a Semiconducting Single-Walled Carbon Nanotubes Interlayer Below the Top Anode. Adv. Mater. Interfaces 2018, 5, 1801155. [Google Scholar] [CrossRef]
- Sokolov, P.M.; Zvaigzne, M.A.; Krivenkov, V.A.; Litvin, A.P.; Baranov, A.V.; Fedorov, A.V.; Samokhvalov, P.S.; Nabiev, I.R. Graphene–quantum dot hybrid nanostructures with controlled optical and photoelectric properties for solar cell applications. Russ. Chem. Rev. 2019, 88, 370–386. [Google Scholar] [CrossRef]
- Martín-García, B.; Polovitsyn, A.; Prato, M.; Moreels, I. Efficient charge transfer in solution-processed PbS quantum dot-reduced graphene oxide hybrid materials. J. Mater. Chem. C 2015, 3, 7088–7095. [Google Scholar] [CrossRef] [Green Version]
- Babaev, A.A.; Parfenov, P.S.; Onishchuk, D.A.; Dubavik, A.; Cherevkov, S.A.; Rybin, A.V.; Baranov, M.A.; Baranov, A.V.; Litvin, A.P.; Fedorov, A.V. Functionalized rGO interlayers improve the fill factor and current density in PbS QDs-based solar cells. Materials (Basel) 2019, 12, 4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugi, M. Langmuir-Blodgett films-a course Towards Molecular Electronics: A Review. J. Mol. Electron. 1985, 1, 3–7. [Google Scholar]
- Dabbousi, B.O.; Murray, C.B.; Rubner, M.F.; Bawendi, M.G. Langmuir-Blodgett Manipulation of Size-Selected CdSe Nanocrystallites. Chem. Mater. 1994, 6, 216–219. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Ushakova, E.V.; Litvin, A.P.; Parfenov, P.S.; Fedorov, A.V.; Artemyev, M.; Prudnikau, A.V.; Rukhlenko, I.D.; Baranov, A.V. Anomalous size-dependent decay of low-energy luminescence from PbS quantum dots in colloidal solution. ACS Nano 2012, 6, 8913–8921. [Google Scholar] [CrossRef]
- Litvin, A.P.; Parfenov, P.S.; Ushakova, E.V.; Fedorov, A.V.; Artemyev, M.V.; Prudnikau, A.V.; Cherevkov, S.A.; Rukhlenko, I.D.; Baranov, A.V. Size-dependent room-temperature luminescence decay from PbS quantum dots. Proc. Nanophotonics Micro/Nano Opt. 2012, 8564, 85641Z. [Google Scholar]
- Lan, X.; Voznyy, O.; García De Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% Certified Colloidal Quantum Dot Solar Cells Via Solvent-Polarity-Engineered Halide Passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Aqoma, H.; Jang, S.Y. Solid-state-ligand-exchange free quantum dot ink-based solar cells with an efficiency of 10.9%. Energy Environ. Sci. 2018, 11, 1603–1609. [Google Scholar] [CrossRef]
- Gorbachev, I.A.; Goryacheva, I.Y.; Glukhovskoy, E.G. Investigation of Multilayers Structures Based on the Langmuir-Blodgett Films of CdSe/ZnS Quantum Dots. Bionanoscience 2016, 6, 153–156. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Y.; Liu, Z.; Han, L.; Shi, G.; Fang, H.; Chen, J.; Ye, X.; Chen, S.; Yang, F.; et al. High-Efficiency PbS Quantum-Dot Solar Cells with Greatly Simplified Fabrication Processing via “Solvent-Curing”. Adv. Mater. 2018, 30, e1707572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parfenov, P.S.; Litvin, A.P.; Ushakova, E.V.; Fedorov, A.V.; Baranov, A.V.; Berwick, K. Note: Near infrared spectral and transient measurements of PbS quantum dots luminescence. Rev. Sci. Instrum. 2013, 84, 116104. [Google Scholar] [CrossRef]
- Litvin, A.P.; Skurlov, I.D.; Korzhenevskii, I.G.; Dubavik, A.; Cherevkov, S.A.; Sokolova, A.V.; Parfenov, P.S.; Onishchuk, D.A.; Zakharov, V.V.; Ushakova, E.V.; et al. Ternary Composites with PbS Quantum Dots for Hybrid Photovoltaics. J. Phys. Chem. C 2019, 123, 3115–3121. [Google Scholar] [CrossRef]
- Brown, P.R.; Kim, D.; Lunt, R.R.; Zhao, N.; Bawendi, M.G.; Grossman, J.C.; Bulović, V. Energy level modification in lead sulfide quantum dot thin films through ligand exchange. ACS Nano 2014, 8, 5863–5872. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Kotov, N.A.; O’Brien, S.; Murray, C.B. Structural diversity in binary nanoparticle superlattices. Nature 2006, 439, 55–59. [Google Scholar] [CrossRef]
- Ushakova, E.V.; Cherevkov, S.A.; Litvin, A.P.; Parfenov, P.S.; Volgina, D.O.A.; Kasatkin, I.A.; Fedorov, A.V.; Baranov, A.V. Ligand-Dependent Morphology and Optical Properties of Lead Sulfide Quantum Dot Superlattices. J. Phys. Chem. C 2016, 120, 25061–25067. [Google Scholar] [CrossRef]
- Baranov, A.V.; Ushakova, E.V.; Golubkov, V.V.; Litvin, A.P.; Parfenov, P.S.; Fedorov, A.V.; Berwick, K. Self-organization of colloidal PBS quantum dots into highly ordered superlattices. Langmuir 2015, 31, 506–513. [Google Scholar] [CrossRef]
- Ushakova, E.V.; Cherevkov, S.A.; Litvin, A.P.; Parfenov, P.S.; Zakharov, V.V.; Dubavik, A.; Fedorov, A.V.; Baranov, A.V. Optical properties of ordered superstructures formed from cadmium and lead chalcogenide colloidal nanocrystals. Opt. Express 2016, 24, A58. [Google Scholar] [CrossRef]
- Fujihira, Y.; Hachisu, T.; Shitanda, S.; Aikawa, K.; Sugiyama, A.; Mizuno, J.; Shoji, S.; Asahi, T.; Osaka, T. Promotion of Self-Assembly Patterning of FePt Nanoparticles by Tuning the Concentration of Oleylamine/Oleic Acid Surfactants in a Coating Solution. J. Electrochem. Soc. 2016, 163, D171–D174. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Tang, Z. Advanced Inorganic Nanoarchitectures from Oriented Self-Assembly. Adv. Mater. 2016, 28, 1096–1108. [Google Scholar] [CrossRef] [PubMed]






| Sample | Specific Conductance, S/m | Sample | Specific Conductance, S/m |
|---|---|---|---|
| LB (EDT) | 4.3 × 10–4 | Spin-coat. (EDT) | 7.5 × 10–5 |
| LB (TBAI) | 2.7 × 10–4 | Spin-coat. (TBAI) | 7.2 × 10–5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litvin, A.P.; Babaev, A.A.; Parfenov, P.S.; Dubavik, A.; Cherevkov, S.A.; Baranov, M.A.; Bogdanov, K.V.; Reznik, I.A.; Ilin, P.O.; Zhang, X.; et al. Ligand-Assisted Formation of Graphene/Quantum Dot Monolayers with Improved Morphological and Electrical Properties. Nanomaterials 2020, 10, 723. https://doi.org/10.3390/nano10040723
Litvin AP, Babaev AA, Parfenov PS, Dubavik A, Cherevkov SA, Baranov MA, Bogdanov KV, Reznik IA, Ilin PO, Zhang X, et al. Ligand-Assisted Formation of Graphene/Quantum Dot Monolayers with Improved Morphological and Electrical Properties. Nanomaterials. 2020; 10(4):723. https://doi.org/10.3390/nano10040723
Chicago/Turabian StyleLitvin, Aleksandr P., Anton A. Babaev, Peter S. Parfenov, Aliaksei Dubavik, Sergei A. Cherevkov, Mikhail A. Baranov, Kirill V. Bogdanov, Ivan A. Reznik, Pavel O. Ilin, Xiaoyu Zhang, and et al. 2020. "Ligand-Assisted Formation of Graphene/Quantum Dot Monolayers with Improved Morphological and Electrical Properties" Nanomaterials 10, no. 4: 723. https://doi.org/10.3390/nano10040723
APA StyleLitvin, A. P., Babaev, A. A., Parfenov, P. S., Dubavik, A., Cherevkov, S. A., Baranov, M. A., Bogdanov, K. V., Reznik, I. A., Ilin, P. O., Zhang, X., Purcell-Milton, F., Gun’ko, Y. K., Fedorov, A. V., & Baranov, A. V. (2020). Ligand-Assisted Formation of Graphene/Quantum Dot Monolayers with Improved Morphological and Electrical Properties. Nanomaterials, 10(4), 723. https://doi.org/10.3390/nano10040723