Titanium-Doped P-Type WO3 Thin Films for Liquefied Petroleum Gas Detection
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
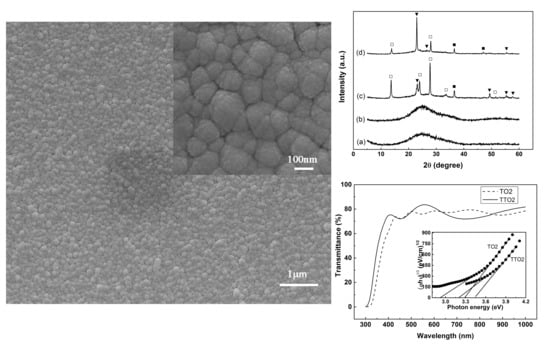
3.1. Structure Characterization
3.2. Energy Band Structure of the TTO and TTO2
3.3. Gas Sensing Performances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, X.; Chen, J.; Yang, J.; Bai, P.; Li, Z.; Wang, Z.L. Ultrathin, rollable, paper-based triboelectric nanogenerator for acoustic energy harvesting and self-powered sound recording. ACS Nano 2015, 9, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tao, C.; Fan, X.; Chen, J. Progress in triboelectric nanogenerators as self-powered smart sensors. J. Mater. Res. 2017, 32, 1628–1646. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, J.; Liu, Y.; Yang, W.; Su, Y.; Wang, Z.L. Triboelectrification-based organic film nanogenerator for acoustic energy harvesting and self-powered active acoustic sensing. ACS Nano 2014, 8, 2649–2657. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Su, Y.; Jing, Q.; Li, Z.; Yi, F.; Wen, X.; Wang, Z.; Wang, Z.L. Eardrum-inspired active sensors for self-powered cardiovascular system characterization and throat-attached anti-interference voice recognition. Adv. Mater. 2015, 27, 1316–1326. [Google Scholar] [CrossRef]
- Meng, K.; Zhao, S.; Zhou, Y.; Wu, Y.; Zhang, S.; He, Q.; Wang, X.; Zhou, Z.; Fan, W.; Tan, X.; et al. A wireless textile based sensor system for self-powered personalized health care. Matter 2020, 2, 896–907. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Li, X.; Zhou, Z.; Meng, K.; Wei, W.; Yang, J.; Wang, Z.L. Triboelectric nanogenerator enabled body sensor network for self-powered human heart-rate monitoring. ACS Nano 2017, 11, 8830–8837. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xie, G.; Chen, J.; Du, H.; Zhang, H.; Yuan, Z.; Ye, Z.; Du, X.; Tai, H.; Jiang, Y. Reduced graphene oxide–polyethylene oxide hybrid films for toluene sensing at room temperature. RSC Adv. 2016, 6, 97840–97847. [Google Scholar] [CrossRef]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, F.; Zhao, S.; Lv, X.; Zhou, Y.; Xiang, S.; Xu, S.; Li, Y.; Chen, G.; Tao, C.; et al. Photo-rechargeable fabrics as sustainable and robust power sources for wearable bioelectronics. Matter 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, Y.; Bick, M.; Chen, J. Smart textiles for electricity generation. Chem. Rev. 2020, 120. [Google Scholar] [CrossRef]
- Yan, C.; Gao, Y.; Zhao, S.; Zhang, S.; Zhou, Y.; Deng, W.; Li, Z.; Jiang, G.; Jin, L.; Tian, G.; et al. A linear-to-rotary hybrid nanogenerator for high-performance wearable biomechanical energy harvesting. Nano Energy 2020, 67, 104235. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.L. Reviving vibration energy harvesting and self-powered sensing by a triboelectric nanogenerator. Joule 2017, 1, 480–521. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Zhang, N.; Zou, H.; Liu, R.; Tao, C.; Fan, X.; Wang, Z.L. Micro-cable structured textile for simultaneously harvesting solar and mechanical energy. Nat. Energy 2016, 1, 16138. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Yang, W.; Jing, Q.; Bai, P.; Yang, Y.; Hou, T.C.; Wang, Z.L. Harmonic-resonator-based triboelectric nanogenerator as a sustainable power source and a self-powered active vibration sensor. Adv. Mater. 2013, 25, 6094–6099. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Waworuntu, E.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Wang, J.; Wang, B.; Yang, T.; Yang, B.; Xie, G.; Zhou, Y.; Zhang, S.; Tai, H.; Jiang, Y.; et al. Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 2020, 14. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Yang, J.; Jing, Q.; Bai, P.; Yang, W.; Qi, X.; Su, Y.; Wang, Z.L. Personalized keystroke dynamics for self-powered human-machine interfacing. ACS Nano 2015, 9, 105. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, J.; Li, X.; Wu, Y.; Wei, W.; Liu, J.; Chen, J.; Yang, J. Large-scale washable smart textile based on triboelectric nanogenerator array for self-powered sleeping monitoring. Adv. Funct. Mater. 2018, 28, 1704112. [Google Scholar] [CrossRef]
- Yan, C.; Deng, W.; Jin, L.; Yang, T.; Wang, Z.; Chu, X.; Su, H.; Chen, J.; Yang, W. Epidermis inspired ultrathin 3D cellular sensors array for self-powered biomedical monitoring. ACS Appl. Mater. Interfaces 2018, 10, 48. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Yang, J.; Su, Y.; Fan, X.; Wu, Y.; Yu, C.; Wang, Z.L. β-cyclodextrin enhanced triboelectricfication for self-powered phenol detection and electrochemical degradation. Energy Environ. Sci. 2015, 8, 887–896. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Wen, X.; Jing, Q.; Yang, J.; Su, Y.; Zhu, G.; Wu, W.; Wang, Z.L. Triboelectrification based motion sensor for human-machine interfacing. ACS Appl. Mater. Interfaces 2014, 6, 10. [Google Scholar] [CrossRef]
- Meng, K.; Chen, J.; Li, X.; Fan, W.; Zhou, Z.; Fan, X.; Zhang, Y.; Yang, J.; Wang, Z.L. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv. Funct. Mater. 2019, 29, 1806388. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Su, Y.; Chen, J.; Adams, K.; Lee, S.; Hu, C.; Wang, Z.L. Triboelectric nanogenerator for harvesting vibration energy in full space and as self-powered acceleration sensor. Adv. Funct. Mater. 2014, 24, 1401. [Google Scholar] [CrossRef]
- Wu, Y.; Jing, Q.; Chen, J.; Bai, P.; Bai, J.; Zhu, G.; Su, Y.; Wang, Z.L. A self-powered angle measurement sensor based on TENG. Adv. Funct. Mater. 2015, 25, 2166. [Google Scholar] [CrossRef]
- Yu, R.; Pan, C.; Chen, J.; Zhu, G.; Wang, Z.L. Enhanced performance of a ZnO nanowire-based self-powered glucose sensor by piezotronic effect. Adv. Funct. Mater. 2013, 23, 5868. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, G.; Yang, W.; Yang, J.; Chen, J.; Jing, Q.; Wu, Z.; Jiang, Y.; Wang, Z.L. Triboelectric sensor for self-powered tracking of object motion inside tubing. ACS Nano 2014, 8, 3843–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, W.; Zhang, T.; Jing, Q.; Chen, J.; Zhou, Y.S.; Bai, P.; Wang, Z.L. Self-powered, ultrasensitive, flexible tactile sensors based on contact electrification. Nano Lett. 2014, 14, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kuang, X.; Deng, J.; Wang, Y.C.; Wang, A.C.; Ding, W.; Lai, Y.C.; Chen, J.; Wang, P.; Lin, Z.; et al. Shape memory polymers for body motion energy harvesting and self-powered mechano-sensing. Adv. Mater. 2018, 30, 1705195. [Google Scholar] [CrossRef]
- Bai, P.; Zhu, G.; Jing, Q.; Wu, Y.; Yang, J.; Chen, J.; Ma, J.; Zhang, G.; Wang, Z.L. Transparent and flexible barcode based on sliding electrification for self-powered identification systems. Nano Energy 2015, 12, 278. [Google Scholar] [CrossRef]
- Wang, Z.L.; Lin, L.; Chen, J.; Niu, S.; Zi, Y. Triboelectric Nanogenerators; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
- Li, Z.; Chen, J.; Guo, H.; Fan, X.; Wen, Z.; Yeh, M.H.; Yu, C.; Cao, X.; Wang, Z.L. Triboelectrification enabled self-powered detection and removal of heavy metal ions in wastewater. Adv. Mater. 2016, 28, 2983. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Triboelectric Nanogenerators; Georgia Institute of Technology: Atlanta, GA, USA, 2016; Available online: http://hdl.handle.net/1853/54956 (accessed on 18 March 2020).
- Wen, Z.; Chen, J.; Yeh, M.-H.; Guo, H.; Li, Z.; Fan, X.; Zhang, T.; Zhu, L.; Wang, Z.L. Blow-driven triboelectric nanogenerator as an active alcohol breath analyzer. Nano Energy 2015, 16, 38–46. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Su, Y.; Chen, J.; Hu, C.; Wu, Z.; Liu, Y.; Ping Wong, C.; Bando, Y.; Wang, Z.L. Triboelectric nanogenerator as self-powered active sensors for detecting liquid/gaseous water/ethanol. Nano Energy 2013, 2, 693–701. [Google Scholar] [CrossRef]
- Akiyama, M.; Tamaki, J.; Miura, N.; Yamazoe, N. Tungsten oxide-based semiconductor sensor highly sensitive to NO and NO2. Chem. Lett. 1991, 1991, 1611–1614. [Google Scholar] [CrossRef]
- Tamaki, J.; Hashishin, T.; Uno, Y.; Dao, D.V.; Sugiyama, S. Ultrahigh-sensitive WO3 nanosensor with interdigitated Au nanoelectrode for NO2 detection. Sens. Actuators B 2008, 132, 234–238. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Wang, B.; Yang, T.; Yang, B.; Xie, G.; Zhou, Y.; Zhang, S.; Tai, H.; Cai, Z.; et al. Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 2020, 14. [Google Scholar] [CrossRef]
- Xue, D.; Wang, J.; Wang, Y.; Sun, G.; Cao, J.; Bala, H.; Zhang, Z. Enhanced methane sensing properties of WO3 nanosheets with dominant exposed (200) facet via loading of SnO2 nanoparticles. Nanomaterials 2019, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chu, X.; Gong, M. Gas-sensing properties of sensors based on single crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B Chem. 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, W.; Fan, A.; Wei, D.; Liu, W.; Cong, H.; Shen, Y.; Meng, D.; San, X. Highly sensitive hydrogen sensors based on SnO2 nanomaterials with different morphologies. Int. J. Hydrogen Energy 2015, 40, 15773–15779. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Luo, P.; Yu, Y.; Chen, D.; Li, X. Incorporating N atoms into SnO2 nanostructure as an approach to enhance gas sensing property for acetone. Nanomaterials 2019, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, M.; Yu, J.; Arsat, R.; Kalantar-zadeh, K.; Comini, E.; Ferroni, M.; Sberveglieri, G.; Wlodarski, W. Reversed bias Pt/nanostructured ZnO Schottky diode with enhanced electric field for hydrogen sensing. Sens. Actuators B 2010, 146, 507–512. [Google Scholar] [CrossRef]
- Wang, L.W.; Kang, Y.F.; Liu, X.H.; Zhang, S.M.; Huang, W.P.; Wang, S.R. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Ling, Z.; Leach, C.; Freer, R. NO2 sensitivity of a heterojunction sensor based on WO3 and doped SnO2. J. Eur. Ceram. Soc. 2003, 23, 1881–1891. [Google Scholar] [CrossRef]
- Ferroni, M.; Carotta, M.C.; Guidi, V.; Martinelli, G.; Ronconi, F.; Richard, O.; Van Dyck, D.; Van Landuyt, J. Structural characterization of Nb-TiO2 nanosized thick-films for gas sensing application. Sens. Actuators B 2000, 68, 140–145. [Google Scholar] [CrossRef]
- Long, H.; Zeng, W.; Zhang, H. Synthesis of WO3 and its gas sensing: A review. J. Mater. Sci. 2015, 26, 4698–4707. [Google Scholar] [CrossRef]
- Meng, D.; Yamazaki, T.; Shen, Y.; Liu, Z.; Kikuta, T. Preparation of WO3 nanoparticles and application to NO2 sensor. Appl. Surf. Sci. 2009, 256, 1050–1053. [Google Scholar] [CrossRef]
- Wei, S.; Xing, Y.; Li, Y.; Zhao, Y.; Du, W.; Zhou, M. Preparation and gas sensing properties of flower-like WO3 hierarchical architecture. Vacuum 2016, 129, 13–19. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Fardindoost, S.; Zad, A.I. Fabrication of Pd doped WO3 nanofiber as hydrogen sensor. Polymers 2013, 5, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Anand, K.; Kohli, N.; Kaur, A.; Singh, R.C. Temperature dependent selective detection of hydrogen and acetone using Pd doped WO3/Reduced graphene oxide nanocomposite. Chem. Phys. Lett. 2018, 701, 115–125. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, C.W. Enhanced ferroelectricity in Ti-doped multiferroic BiFeO3 thin films. Appl. Phys. Lett. 2006, 89, 052903. [Google Scholar] [CrossRef]
- Lee, S.H.; Cheong, H.M.; Park, N.G.; Tracy, C.E.; Mascarenhas, A.; Benson, D.K.; Deb, S.K. Raman spectroscopic studies of Ni–W oxide thin films. Solid State Ion. 2001, 140, 135–139. [Google Scholar] [CrossRef]
- He, X.L.; Li, J.P.; Gao, X.G.; Wang, L. NO2 sensing characteristics of WO3 thin film microgas sensor. Sens. Actuators B 2003, 93, 463–467. [Google Scholar] [CrossRef]
- Lozzi, L.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Cantalini, C. The influence of air and vacuum thermal treatments on the NO2 gas sensitivity of WO3 thin films prepared by thermal evaporation. Thin Solid Film. 2001, 391, 224–228. [Google Scholar] [CrossRef]
- Pal, S.; Jacob, C. Novel technique for large scale production of spherical tungsten oxide nanoparticles. J. Mater. Sci. 2006, 41, 5429–5432. [Google Scholar] [CrossRef]
- Bennett, R.A.; Mulley, J.S.; Newton, M.A.; Surman, M. Spectroscopy of ultrathin epitaxial rutile TiO2(110) films grown on W(100). J. Chem. Phys. 2007, 127, 084707. [Google Scholar] [CrossRef]
- Hutchins, M.G.; Abu-Alkhair, O.; El-Nahass, M.M.; El-Hady, K.A. Structural and optical characterisation of thermally evaporated tungsten trioxide (WO3) thin films. Mater. Chem. Phys. 2006, 98, 401–405. [Google Scholar] [CrossRef]
- Shimizu, Y.; Egashira, M. Basic aspects and challenges of semiconductor gas sensors. MRS Bull. 1999, 24, 18–24. [Google Scholar] [CrossRef]






| Sample | Type | Carrier Density (cm−3) | Resistivity (Ωcm) | Mobility (cm2V−1s−1) |
|---|---|---|---|---|
| TO2 | n | 3.729 × 1011 | 1.579 × 104 | 1.060 × 103 |
| TTO2 | p | 9.227 × 1012 | 5.223 × 103 | 1.295 × 102 |
| Sample | Phase | a (Å) | b (Å) | c (Å) |
|---|---|---|---|---|
| TO2 | WO3 | 7.300 | 7.542 | 7.610 |
| TTO2 | WO3 | 7.351 | 7.599 | 7.656 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Shi, X.; Chen, K.; Yang, X.; Chen, J. Titanium-Doped P-Type WO3 Thin Films for Liquefied Petroleum Gas Detection. Nanomaterials 2020, 10, 727. https://doi.org/10.3390/nano10040727
He Y, Shi X, Chen K, Yang X, Chen J. Titanium-Doped P-Type WO3 Thin Films for Liquefied Petroleum Gas Detection. Nanomaterials. 2020; 10(4):727. https://doi.org/10.3390/nano10040727
Chicago/Turabian StyleHe, Yuzhenghan, Xiaoyan Shi, Kyle Chen, Xiaohong Yang, and Jun Chen. 2020. "Titanium-Doped P-Type WO3 Thin Films for Liquefied Petroleum Gas Detection" Nanomaterials 10, no. 4: 727. https://doi.org/10.3390/nano10040727
APA StyleHe, Y., Shi, X., Chen, K., Yang, X., & Chen, J. (2020). Titanium-Doped P-Type WO3 Thin Films for Liquefied Petroleum Gas Detection. Nanomaterials, 10(4), 727. https://doi.org/10.3390/nano10040727