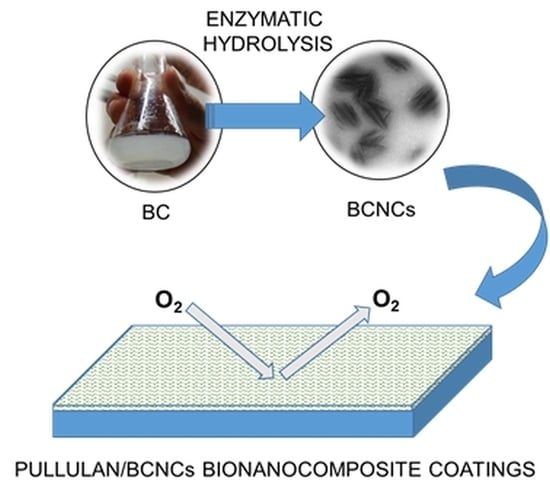
Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prodution of Macro-Sized Bacterial Cellulose (BC)
2.2. Enzymatic Hydrolysis of BC
2.3. Pullulan/Bacterial Cellulose Nanocrystals (BCNCs) Nanocomposite Coating Preparation
2.4. Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Kinetics of the Enzymatic Hydrolysis
3.2. Morphology and Size Distribution of Hydrolyzed BC
3.3. Oxygen Barrier Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; Borges, M.; Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic acid bacteria in the food industry: Systematics, characteristics and applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Holt, B.L.; Stoyanov, S.D.; Pelanb, E.; Paunov, V.N. Novel anisotropic materials from functionalised colloidal cellulose and cellulose derivatives. J. Mater. Chem. 2010, 20, 10058–10070. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lee, S.Y.; Park, K.J.; Park, S.M.; An, H.J.; Hyun, J.M.; Choi, Y.H. Gluconacetobacter sp. gel_SEA623-2, bacterial cellulose producing bacterium isolated from citrus fruit juice. Saudi J. Biol. Sci. 2017, 24, 314–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Ramana, K.V.; Bawa, A.S.; Siddaramaiah. Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Mattoso, L.H.C.; Bernardes-Filho, R.; Wood, D.F.; Orts, W.J. Self-assembled films of cellulose nanofibrils and poly(o-ethoxyaniline). Colloid. Polym. Sci. 2008, 286, 1265–1272. [Google Scholar] [CrossRef]
- Ahola, S.; Turon, X.; Osterberg, M.; Laine, J.; Rojas, O.J. Enzymatic hydrolysis of native cellulose nanofibrils and other cellulose model films: Effect of surface structure. Langmuir 2008, 24, 11592–11599. [Google Scholar] [CrossRef]
- Rabinovich, M.L.; Melnick, M.S.; Bolobova, A.V. The structure and mechanism of action of cellulolytic enzymes. Biochemistry (Moscow) 2002, 67, 850–871. [Google Scholar] [CrossRef] [PubMed]
- Rovera, C.; Ghaani, M.; Santo, N.; Trabattoni, S.; Olsson, R.T.; Romano, D.; Farris, S. Enzymatic hydrolysis in the green production of bacterial cellulose nanocrystals. ACS Sustainable Chem. Eng. 2018, 6, 7725–7734. [Google Scholar] [CrossRef]
- Unalan, I.U.; Boyacı, D.; Ghaani, M.; Trabattoni, S.; Farris, S. Graphene oxide bionanocomposite coatings with high oxygen barrier properties. Nanomaterials 2016, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Magonov, S.N.; Elings, V.; Whangbo, M.-H. Phase imaging and stiffness in tapping-mode atomic force microscopy. Surf. Sci. 1997, 375, L385–L391. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Wahyudi, H.; Agustina, S.; Oudet, F.; Pinem, M.P.; Clausse, D.; Saleh, K.; Guénin, E. Ultrasonic irradiation coupled with microwave treatment for eco-friendly process of isolating bacterial cellulose nanocrystals. Nanomaterials 2016, 8, 859. [Google Scholar] [CrossRef] [Green Version]
- Hafemann, E.; Battisti, R.; Marangoni, C.; Machado, R.A.F. Valorization of royal palm tree agroindustrial waste by isolating cellulose nanocrystals. Carbohydr. Polym. 2019, 218, 188–198. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gidley, M.J.; Gilbert, E.P. Hierarchical architecture of bacterial cellulose and composite plant cell wall polysaccharide hydrogels using small angle neutron scattering. Soft Matter 2016, 12, 1534–1549. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-based films and coatings for food packaging: Present applications, emerging opportunities, and future challenges. J. Appl. Polym. Sci 2014, 131, 40539–40551. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, C.A.; Cerri, G.; Brundu, A.; Farris, S. Microfibrillated cellulose (MFC): Pullulan bionanocomposite films. Cellulose 2014, 21, 4323–4335. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, C.A.; Campanella, G.; Türe, H.; Olsson, R.T.; Farris, S. Microfibrillated cellulose and borax as mechanical, O₂-barrier, and surface-modulating agents of pullulan biocomposite coatings on BOPP. Carbohydr. Polym. 2016, 143, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, C.A.; Castelli, G.; Trabattoni, S.; Farris, S. Influence of colloidal silica nanoparticles on pullulan-coated BOPP film. Food Packag. Shelf Life 2016, 8, 50–55. [Google Scholar] [CrossRef]
- Unalan, I.U.; Wan, C.; Figiel, L.; Olsson, R.T.; Trabattoni, S.; Farris, S. Exceptional oxygen barrier performance of pullulan nanocomposites with ultra-low loading graphene oxide. Nanotechnology 2015, 26, 275703–275713. [Google Scholar] [CrossRef] [PubMed]
- Unalan, I.U.; Boyaci, D.; Trabattoni, S.; Tavazzi, S.; Farris, S. Transparent pullulan/mica nanocomposite coatings with outstanding oxygen barrier proprieties. Nanomaterials 2017, 7, 281. [Google Scholar] [CrossRef]




| Cellulase a/BC b (w/w) | Cellulase a (mg) | Enzyme Unit (U) |
| 1:4 | 2.5 | 16.25 |
| Endo-1,4-β-glucanase c/BC b (v/w) | Endo-1,4-β-glucanase c (μL) | Enzyme Unit (U) |
| 1:4 | 600 | 90 |
| Filler Content | OTR (0% RH) (mL·m−2·24 h−1) | OTR (80% RH) (mL·m−2·24 h−1) |
|---|---|---|
| PET | 120.3 ± 2.1 a | 109.5 ± 1.7 A |
| PET/pullulan | 6.2 ± 0.8 b | 100.73 ± 3.23 A |
| PET/pullulan/BCNCs (EGs) | 0.840 ± 0.12 c | 94.14 ± 2.19 B |
| PET/pullulan/BCNCs (EGs + cellulase) | 0.702 ± 0.09 c | 89.71 ± 2.21 B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovera, C.; Fiori, F.; Trabattoni, S.; Romano, D.; Farris, S. Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry. Nanomaterials 2020, 10, 735. https://doi.org/10.3390/nano10040735
Rovera C, Fiori F, Trabattoni S, Romano D, Farris S. Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry. Nanomaterials. 2020; 10(4):735. https://doi.org/10.3390/nano10040735
Chicago/Turabian StyleRovera, Cesare, Filippo Fiori, Silvia Trabattoni, Diego Romano, and Stefano Farris. 2020. "Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry" Nanomaterials 10, no. 4: 735. https://doi.org/10.3390/nano10040735
APA StyleRovera, C., Fiori, F., Trabattoni, S., Romano, D., & Farris, S. (2020). Enzymatic Hydrolysis of Bacterial Cellulose for the Production of Nanocrystals for the Food Packaging Industry. Nanomaterials, 10(4), 735. https://doi.org/10.3390/nano10040735