Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications
Abstract
:1. Introduction
2. Charged Lipids as pH-Triggering Adjuvant
3. Drugs as pH-Responsive Inducers
4. Ionic Surfactants
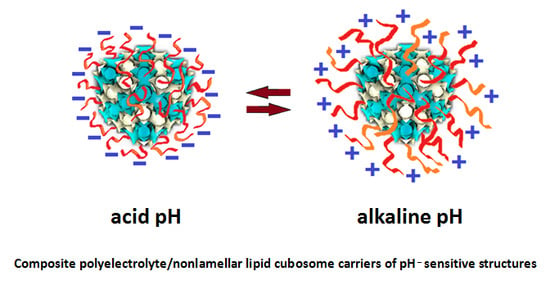
5. Polyelectrolytes in Cubosomes
6. Perspectives of pH-Sensitive Cubosomes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyd, B.J.; Khoo, S.; Whittaker, D.; Davey, G.; Porter, C.J. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tiberg, F.; Johnsson, M. Drug delivery applications of non-lamellar liquid crystalline phases and nanoparticles. J. Drug Deliv. Sci. Technol. 2011, 21, 101–109. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A.-H. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules 2019, 25, 16. [Google Scholar] [CrossRef] [Green Version]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid Crystalline Nanostructures as PEGylated Reservoirs of Omega-3 Polyunsaturated Fatty Acids: Structural Insights toward Delivery Formulations against Neurodegenerative Disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.; Hong, L.; Du, J.D.; Boyd, B.J. Self-Assembled Nanostructured Lipid Systems: Is There a Link between Structure and Cytotoxicity? Adv. Sci. 2018, 6, 1801223. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Kang, Y.-S.; Lee, D.S.; Park, H.-J.; Choi, E.-K.; Oh, Y.-K.; Son, H.-J.; Kim, J.-S. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J. Control. Release 2009, 140, 55–60. [Google Scholar] [CrossRef]
- Masotti, A. Niosomes as candidate bioconjugates for imaging and pH-sensitive drug delivery nanocarriers for rare pediatric tumors. J. Drug Deliv. Sci. Technol. 2013, 23, 22–24. [Google Scholar] [CrossRef]
- Faria, A.R.; Silvestre, O.; Maibohm, C.; Adão, R.M.R.; Silva, B.F.B.; Nieder, J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD(P)H multi-photon fluorescence lifetime imaging. Nano Res. 2018, 12, 991–998. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Nithya, R.; Jerold, P.; Siram, K. Cubosomes of dapsone enhanced permeation across the skin. J. Drug Deliv. Sci. Technol. 2018, 48, 75–81. [Google Scholar] [CrossRef]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: Ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zeng, N.; Gao, X.; Hu, Q.; Song, Q.; Xia, H.; Liu, Z.; Gu, G.; Pang, Z.; Chen, H.; et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: Cellular interaction and in vivo absorption. Int. J. Nanomed. 2012, 7, 3703–3718. [Google Scholar] [CrossRef] [Green Version]
- Swarnakar, N.K.; Thanki, K.; Jain, S. Bicontinuous Cubic Liquid Crystalline Nanoparticles for Oral Delivery of Doxorubicin: Implications on Bioavailability, Therapeutic Efficacy, and Cardiotoxicity. Pharm. Res. 2013, 31, 1219–1238. [Google Scholar] [CrossRef]
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles as Oral Drug Delivery Systems: I. In Vitro Evaluation and Enhanced Oral Bioavailability of the Poorly Water-Soluble Drug Simvastatin. AAPS PharmSciTech 2009, 10, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Dyett, B.P.; Yu, H.; Strachan, J.; Drummond, C.J.; Conn, C.E. Fusion dynamics of cubosome nanocarriers with model cell membranes. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P.; Patrick, C. Self-Assembled Multicompartment Liquid Crystalline Lipid Carriers for Protein, Peptide, and Nucleic Acid Drug Delivery. Accounts Chem. Res. 2011, 44, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Valldeperas, M.; Salis, A.; Barauskas, J.; Tiberg, F.; Arnebrant, T.; Razumas, V.; Monduzzi, M.; Nylander, T. Enzyme encapsulation in nanostructured self-assembled structures: Toward biofunctional supramolecular assemblies. Curr. Opin. Colloid Interface Sci. 2019, 44, 130–142. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Stepanek, P.; Lesieur, S. Multicompartment Lipid Cubic Nanoparticles with High Protein Upload: Millisecond Dynamics of Formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Caffrey, M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J. Control. Release 2005, 107, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Zabara, A.; Mezzenga, R. Controlling molecular transport and sustained drug release in lipid-based liquid crystalline mesophases. J. Control. Release 2014, 188, 31–43. [Google Scholar] [CrossRef]
- Fong, W.-K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. J. Control. Release 2009, 135, 218–226. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Fong, W.-K.; Hanley, T.L.; Thierry, B.; Tilley, A.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Understanding the photothermal heating effect in non-lamellar liquid crystalline systems, and the design of new mixed lipid systems for photothermal on-demand drug delivery. Phys. Chem. Chem. Phys. 2014, 16, 24936–24953. [Google Scholar] [CrossRef]
- Rarokar, N.; Saoji, S.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured Cubosomes in a Thermoresponsive Depot System: An Alternative Approach for the Controlled Delivery of Docetaxel. AAPS PharmSciTech 2015, 17, 436–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Dong, Y.-D.; Hanley, T.L.; Boyd, B.J. Sensitivity of Nanostructure in Charged Cubosomes to Phase Changes Triggered by Ionic Species in Solution. Langmuir 2013, 29, 14265–14273. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.W.; Zhen, G.; Gunatillake, P.; Hartley, P. Salt Induced Lamellar to Bicontinuous Cubic Phase Transitions in Cationic Nanoparticles. J. Phys. Chem. B 2012, 116, 3551–3556. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Tyler, A.; McCarthy, N.L.C.; Parsons, E.S.; Ces, O.; Law, R.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borné, J.; Nylander, T.; Khan, A. Phase Behavior and Aggregate Formation for the Aqueous Monoolein System Mixed with Sodium Oleate and Oleic Acid. Langmuir 2001, 17, 7742–7751. [Google Scholar] [CrossRef]
- Mele, S.; Söderman, O.; Ljusberg-Wahrén, H.; Thuresson, K.; Monduzzi, M.; Nylander, T. Phase behavior in the biologically important oleic acid/sodium oleate/water system. Chem. Phys. Lipids 2018, 211, 30–36. [Google Scholar] [CrossRef]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-Assembled Structures and pKaValue of Oleic Acid in Systems of Biological Relevance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef]
- Yaghmur, A.; Sartori, B.; Rappolt, M. Self-Assembled Nanostructures of Fully Hydrated Monoelaidin–Elaidic Acid and Monoelaidin–Oleic Acid Systems. Langmuir 2012, 28, 10105–10119. [Google Scholar] [CrossRef]
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-Triggered nanostructural transformations in antimicrobial peptide/oleic acid self-assemblies. Biomater. Sci. 2018, 6, 803–812. [Google Scholar] [CrossRef]
- Tran, N.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Giakoumatos, E.C.; Waddington, L.J.; Kirby, N.; Drummond, C.J.; Mulet, X.; Muir, B.W. Nanostructure and cytotoxicity of self-assembled monoolein–capric acid lyotropic liquid crystalline nanoparticles. RSC Adv. 2015, 5, 26785–26795. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the Ordered Nanostructure of Self-Assembled Monoolein and Phytantriol Nanoparticles with Unsaturated Fatty Acids. Langmuir 2018, 34, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-Throughput Screening of Saturated Fatty Acid Influence on Nanostructure of Lyotropic Liquid Crystalline Lipid Nanoparticles. Langmuir 2016, 32, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.; Zheng, Y.; Gong, Y.; Fu, M.; Liu, C.; Xu, L.; Sun, C.C.; Gao, Y.; Qian, S. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019, 9, 6287–6298. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Ahuja, M. Optimization, characterization and evaluation of chitosan-tailored cubic nanoparticles of clotrimazole. Int. J. Boil. Macromol. 2015, 73, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Svensson, O.; Thuresson, K.; Arnebrant, T. Interactions between chitosan-modified particles and mucin-coated surfaces. J. Colloid Interface Sci. 2008, 325, 346–350. [Google Scholar] [CrossRef]
- Mathews, P.D.; Mertins, O. Dispersion of chitosan in liquid crystalline lamellar phase: Production of biofriendly hydrogel of nano cubic topology. Carbohydr. Polym. 2017, 157, 850–857. [Google Scholar] [CrossRef]
- Mathews, P.D.; Patta, A.C.M.F.; Gonçalves, J.V.; Gama, G.D.S.; Garcia, I.T.S.; Mertins, O. Targeted Drug Delivery and Treatment of Endoparasites with Biocompatible Particles of pH-Responsive Structure. Biomacromolecules 2018, 19, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nielsen, H.M.; Müllertz, A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1289–1304. [Google Scholar] [CrossRef]
- Shah, M.H.; Paradkar, A. Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. Int. J. Pharm. 2005, 294, 161–171. [Google Scholar] [CrossRef]
- He, S.; Liu, Z.; Xu, D. Advance in oral delivery systems for therapeutic protein. J. Drug Target. 2018, 27, 283–291. [Google Scholar] [CrossRef]
- Smart, A.L.; Gaisford, S.; Basit, A.W. Oral peptide and protein delivery: Intestinal obstacles and commercial prospects. Expert Opin. Drug Deliv. 2014, 11, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Patta, A.C.F.; Mathews, P.D.; Madrid, R.R.; Rigoni, V.L.; Silva, E.R.; Mertins, O. Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications. Int. J. Boil. Macromol. 2020, 148, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Garamus, V.M.; Mutafchieva, R.; Angelova, A.; Drechsler, M.; Lesieur, S. Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. Soft Matter 2015, 11, 3686–3692. [Google Scholar] [CrossRef] [Green Version]
- Barriga, H.M.G.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Engineering Swollen Cubosomes Using Cholesterol and Anionic Lipids. Langmuir 2019, 35, 16521–16527. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Narayanan, T.; Drechsler, M.; Stepanek, P.; Couvreur, P.; Lesieur, S.; Patrick, C. DNA/Fusogenic Lipid Nanocarrier Assembly: Millisecond Structural Dynamics. J. Phys. Chem. Lett. 2013, 4, 1959–1964. [Google Scholar] [CrossRef]
- Leung, S.S.W.; Leal, C. The stabilization of primitive bicontinuous cubic phases with tunable swelling over a wide composition range. Soft Matter 2019, 15, 1269–1277. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A.-H. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Nakano, M.; Teshigawara, T.; Sugita, A.; Leesajakul, W.; Taniguchi, A.; Kamo, T.; Matsuoka, H.; Handa, T. Dispersions of Liquid Crystalline Phases of the Monoolein/Oleic Acid/Pluronic F127 System. Langmuir 2002, 18, 9283–9288. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R.; Fong, W.-K.; Boyd, B.J. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazaruk, E.; Szlęzak, M.; Gorecka, E.; Bilewicz, R.; Osornio, Y.M.; Uebelhart, P.; Landau, E.M. Design and Assembly of pH-Sensitive Lipidic Cubic Phase Matrices for Drug Release. Langmuir 2014, 30, 1383–1390. [Google Scholar] [CrossRef]
- Osornio, Y.M.; Uebelhart, P.; Bosshard, S.; Konrad, F.; Siegel, J.S.; Landau, E.M. Design and Synthesis of Lipids for the Fabrication of Functional Lipidic Cubic-Phase Biomaterials. J. Org. Chem. 2012, 77, 10583–10595. [Google Scholar] [CrossRef]
- Oka, T.; Hasan, M.; Islam, Z.; Moniruzzaman; Yamazaki, M. Low-pH-Induced Lamellar to Bicontinuous Primitive Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein Membranes. Langmuir 2017, 33, 12487–12496. [Google Scholar] [CrossRef]
- Oka, T.; Tsuboi, T.-A.; Saiki, T.; Takahashi, T.; Alam, J.M.; Yamazaki, M. Initial Step of pH-Jump-Induced Lamellar to Bicontinuous Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein. Langmuir 2014, 30, 8131–8140. [Google Scholar] [CrossRef]
- Oka, T.; Saiki, T.; Alam, J.M.; Yamazaki, M. Activation Energy of the Low-pH-Induced Lamellar to Bicontinuous Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein. Langmuir 2016, 32, 1327–1337. [Google Scholar] [CrossRef]
- Nazaruk, E.; Gorecka, E.; Osornio, Y.M.; Landau, E.M.; Bilewicz, R. Charged additives modify drug release rates from lipidic cubic phase carriers by modulating electrostatic interactions. J. Electroanal. Chem. 2018, 819, 269–274. [Google Scholar] [CrossRef]
- Fong, C.; Zhai, J.; Drummond, C.J.; Tran, N. Micellar Fd3m cubosomes from monoolein—Long chain unsaturated fatty acid mixtures: Stability on temperature and pH response. J. Colloid Interface Sci. 2020, 566, 98–106. [Google Scholar] [CrossRef]
- Rahanyan-Kägi, N.; Aleandri, S.; Speziale, C.; Mezzenga, R.; Landau, E.M. Stimuli-Responsive Lipidic Cubic Phase: Triggered Release and Sequestration of Guest Molecules. Chem. - A Eur. J. 2014, 21, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Salentinig, S.; Tangso, K.J.; Hawley, A.; Boyd, B.J. pH-Driven Colloidal Transformations Based on the Vasoactive Drug Nicergoline. Langmuir 2014, 30, 14776–14781. [Google Scholar] [CrossRef] [PubMed]
- Zabara, A.; Negrini, R.; Onaca-Fischer, O.; Mezzenga, R. Perforated Bicontinuous Cubic Phases with pH-Responsive Topological Channel Interconnectivity. Small 2013, 9, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Rappolt, M.; Østergaard, J.; Larsen, C.; Larsen, S. Characterization of Bupivacaine-Loaded Formulations Based on Liquid Crystalline phases and Microemulsions: The Effect of Lipid Composition. Langmuir 2012, 28, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.R.; Immich, M.F.; Lundberg, D.; Poletto, F.; Loh, W. Physiological neutral pH drives a gradual lamellar-to-reverse cubic-to-reverse hexagonal phase transition in phytantriol-based nanoparticles. Colloids Surf. B Biointerfaces 2019, 177, 204–210. [Google Scholar] [CrossRef]
- Hubčík, L.; Funari, S.S.; Pullmannová, P.; Devínsky, F.; Uhrikova, D. Stimuli responsive polymorphism of C12NO/DOPE/DNA complexes: Effect of pH, temperature and composition. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 1127–1138. [Google Scholar] [CrossRef]
- Kluzek, M.; Wang, S.; Chen, R.; Marques, C.M.; Thalmann, F.; Seddon, J.; Schmutz, M.; Tyler, A. Influence of a pH-sensitive polymer on the structure of monoolein cubosomes. Soft Matter 2017, 13, 7571–7577. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Chrysostomou, V.; Pispas, S.; Chrysina, E.D.; Forys, A.; Otulakowski, L.; Trzebicka, B.; Demetzos, C. Stimuli-Responsive Lyotropic Liquid Crystalline Nanosystems with Incorporated Poly(2-Dimethylamino Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate) Amphiphilic Block Copolymer. Polymers 2019, 11, 1400. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.J.; Kim, J.-C. Controlled Release from Monoolein Cubic Phase by Complexation between Acidic Proteinoid and Basic Proteinoid. J. Dispers. Sci. Technol. 2012, 33, 605–610. [Google Scholar] [CrossRef]
- Kwon, T.K.; Kim, J.-C. Complex Coacervation-Controlled Release from Monoolein Cubic Phase Containing Silk Fibroin and Alginate. Biomacromolecules 2011, 12, 466–471. [Google Scholar] [CrossRef]
- Crisci, A.; Hay, D.N.T.; Seifert, S.; Firestone, M.A. pH- and Ionic-Strength-Induced Structural Changes in Poly(acrylic acid)-Lipid-Based Self-Assembled Materials. Macromol. Symp. 2009, 281, 126–134. [Google Scholar] [CrossRef]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Gorecka, E.; Landau, E.M.; Bilewicz, R.; Lau, E.M. Lyotropic Cubic Phases for Drug Delivery: Diffusion and Sustained Release from the Mesophase Evaluated by Electrochemical Methods. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Fioravanti, M.; Dolezal, T.; Logina, I.; Milanov, I.; Popescu, D.C.; Solomon, A. Therapeutic use of nicergoline. Clin. Drug Investig. 2008, 28, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Buur, A.; Larsen, C. Bioreversible quaternary N-acyloxymethyl derivatives of the tertiary amines bupivacaine and lidocaine—Synthesis, aqueous solubility and stability in buffer, human plasma and simulated intestinal fluid. Eur. J. Pharm. Sci. 2005, 24, 433–440. [Google Scholar] [CrossRef]
- Astolfi, P.; Giorgini, E.; Adamo, F.C.; Vita, F.; Logrippo, S.; Francescangeli, O.; Pisani, M. Effects of a cationic surfactant incorporation in phytantriol bulk cubic phases and dispersions loaded with the anticancer drug 5-fluorouracil. J. Mol. Liq. 2019, 286, 110954. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Pispas, S. Stimuli-responsive amphiphilic PDMAEMA-b-PLMA copolymers and their cationic and zwitterionic analogs. J. Polym. Sci. Part A Polym. Chem. 2017, 56, 598–610. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. Complexation-responsive monoolein cubic phase containing extract of Bambusae Caulis in Taeniam. Int. J. Polym. Mater. 2018, 69, 44–52. [Google Scholar] [CrossRef]
- Anghel, D.F.; Toca-Herrera, J.L.; Winnik, F.M.; Rettig, W.; Klitzing, R.V. Steady-State Fluorescence Investigation of Pyrene-Labeled Poly(Acrylic Acid)s in Aqueous Solution and in the Presence of Sodium Dodecyl Sulfate. Langmuir 2002, 18, 5600–5606. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Sieval, A.B.; Fleer, G.J.; Stuart, M.A.C. Polyacrylic Acid Brushes: Surface Pressure and Salt-Induced Swelling. Langmuir 2000, 16, 8324–8333. [Google Scholar] [CrossRef]
- Ahmad, A.; Evans, H.M.; Ewert, K.K.; George, C.X.; Samuel, C.E.; Safinya, C.R. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: Lipid-DNA complexes for gene delivery. J. Gene Med. 2005, 7, 739–748. [Google Scholar] [CrossRef]
- Ewert, K.K.; Evans, H.M.; Bouxsein, N.F.; Safinya, C.R. Dendritic Cationic Lipids with Highly Charged Headgroups for Efficient Gene Delivery. Bioconjugate Chem. 2006, 17, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Hinton, T.M.; Muir, B.W.; Shi, S.; Tizard, M.; McLean, K.M.; Hartley, P.; Gunatillake, P. Glycerol Monooleate-Based Nanocarriers for siRNA Delivery in Vitro. Mol. Pharm. 2012, 9, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: High throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter 2011, 7, 4768. [Google Scholar] [CrossRef]
- Chong, J.; Mulet, X.; Keddie, D.J.; Waddington, L.; Mudie, S.T.; Boyd, B.J.; Drummond, C.J. Novel Steric Stabilizers for Lyotropic Liquid Crystalline Nanoparticles: PEGylated-Phytanyl Copolymers. Langmuir 2014, 31, 2615–2629. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, B. Polymeric Modification and Its Implication in Drug Delivery: Poly-ε-caprolactone (PCL) as a Model Polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.B.; Pakalapati, H.; Khalid, M.; Walvekar, R.; Siddiqui, H. Natural and synthetic biocompatible and biodegradable polymers. In Biodegradable and Biocompatible Polymer Composites; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 3–32. [Google Scholar]
- Mathews, P.D.; Mertins, O. Chitosan and lipid composites as versatile biomedical material. In Materials for Biomedical Engineering; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 259–291. [Google Scholar]
- Mertins, O.; Lobo, S.E.; Mathews, P.D.; Han, S.W. Interaction of pDNA with reverse phase chitosome. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 76–82. [Google Scholar] [CrossRef]
- Gonçalves, M.C.F.; Mertins, O.; Pohlmann, A.R.; Da Silveira, N.P.; Guterres, S.S. Chitosan coated liposomes as an innovative nanocarrier for drugs. J. Biomed. Nanotechnol. 2012, 8, 240–250. [Google Scholar] [CrossRef]
- Marón, L.B.; Covas, C.P.; Da Silveira, N.P.; Pohlmann, A.R.; Mertins, O.; Tatsuo, L.N.; Sant´anna, O.A.B.; Moro, A.M.; Takata, C.S.; De Araujo, P.S.; et al. LUVs Recovered with Chitosan: A New Preparation for Vaccine Delivery. J. Liposome Res. 2007, 17, 155–163. [Google Scholar] [CrossRef]
- Mertins, O.; Mathews, P.D.; Gomide, A.B.; Baptista, M.S.; Itri, R. Effective protection of biological membranes against photo-oxidative damage: Polymeric antioxidant forming a protecting shield over the membrane. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2180–2187. [Google Scholar] [CrossRef] [Green Version]
- Garcia, B.B.M.; Mertins, O.; Silva, E.R.; Mathews, P.D.; Han, S.W. Arginine-modified chitosan complexed with liposome systems for plasmid DNA delivery. Colloids Surf. B Biointerfaces 2020, accepted. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109006. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Natesan, S.; Kandasamy, R. pH responsive delivery of lumefantrine with calcium phosphate nanoparticles loaded lipidic cubosomes for the site specific treatment of lung cancer. Chem. Phys. Lipids 2019, 224, 104763. [Google Scholar] [CrossRef] [PubMed]
- Gaware, S.A.; Rokade, K.A.; Kale, S. Silica-chitosan nanocomposite mediated pH-sensitive drug delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 345–351. [Google Scholar] [CrossRef]
- Xie, C.; Tian, T.-C.; Yu, S.-T.; Li, L. pH-sensitive hydrogel based on carboxymethyl chitosan/sodium alginate and its application for drug delivery. J. Appl. Polym. Sci. 2018, 136. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Huang, Z.; Chen, J.; Cui, Y.; Niu, B.; Huang, Y.; Pan, X.; Wu, C. Smart phase transformation system based on lyotropic liquid crystalline@hard capsules for sustained release of hydrophilic and hydrophobic drugs. Drug Deliv. 2020, 27, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, H.M.; Elnaggar, Y.S.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Driever, C.D.; Mulet, X.; Waddington, L.J.; Postma, A.; Thissen, H.; Caruso, F.; Drummond, C.J. Layer-by-Layer Polymer Coating on Discrete Particles of Cubic Lyotropic Liquid Crystalline Dispersions (Cubosomes). Langmuir 2013, 29, 12891–12900. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.; Abdelmonsif, D.A.; Abdallah, O.Y. Layer-by-layer-coated lyotropic liquid crystalline nanoparticles for active tumor targeting of rapamycin. Nanomedicine 2016, 11, 2975–2996. [Google Scholar] [CrossRef]
- Men, W.; Zhu, P.; Dong, S.; Liu, W.; Zhou, K.; Bai, Y.; Liu, X.; Gong, S.; Zhang, S.-G. Layer-by-layer pH-sensitive nanoparticles for drug delivery and controlled release with improved therapeutic efficacy in vivo. Drug Deliv. 2020, 27, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Ruela, A.L.M.; Carvalho, F.C.; Pereira, G.R.; Information, P.E.K.F.C. Exploring the Phase Behavior of Monoolein/Oleic Acid/Water Systems for Enhanced Donezepil Administration for Alzheimer Disease Treatment. J. Pharm. Sci. 2016, 105, 71–77. [Google Scholar] [CrossRef]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Qiu, Y.; Yang, W.; Guo, Y.; Hou, H.; Liu, Z.; Zhao, X. Charge reversible and biodegradable nanocarriers showing dual pH-/reduction-sensitive disintegration for rapid site-specific drug delivery. Colloids Surf. B Biointerfaces 2018, 169, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Jog, R.; Unachukwu, K.; Burgess, D.J. Formulation design space for stable, pH sensitive crystalline nifedipine nanoparticles. Int. J. Pharm. 2016, 514, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Eslami, M.; Zangabad, P.S.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2016, 8, 696–716. [Google Scholar] [CrossRef] [Green Version]







| Lipids | Additives | Preparation Methodology | Studied pH Values | Liquid Crystalline Phases | Perspective for Application | Refs. |
|---|---|---|---|---|---|---|
| Monoolein Oleic acid | Brucea javanica oil Pluronic F127 PBS Doxorubicin | Melting 60 °C Stirring High-pressure homogenization | 7.4 6.8 5.3 | HII Pn3m, Im3m microemulsion | Dual-drug (BJO, DOX) delivery/cancer inhibition (in vitro tested) | [59] |
| Monoolein Oleic acid | Pluronic F127 PBS | Heating 80 °C Homogenization High pressure | 6.0 7.0 | HII Im3m | Drug delivery (perspective) | [60] |
| Monolinolein Linoleic acid | Phloroglucinol | Hydration Heating Vortex mixing | 2.0 7.0 | HII Im3m | Oral drug delivery (perspective) | [61] |
| Monolinolein Pyridinylmethyl linoleate | Doxorubicin | Hydration Heating Vortex mixing | 5.5 7.4 | Pn3m HII | Tumor-targeted delivery (in vitro tested) | [62] |
| Monoolein 2-hydroxyoleic Acid | Pluronic F127 PBS | Ultrasonication | 2.0; 3.0 3.5; 4.0; 4.5 5.0; 6.0; 7.4 | Pn3m, HII Pn3m, Im3m Lamellar | Tumor-targeted delivery (perspective) | [63] |
| Monoolein Phytantriol “Lipid 1” | Doxorubicin | Melting Hydration Centrifugation | 5.8 7.5 9.0 | Pn3m Pn3m Pn3m | Drug delivery (perspective) | [64] |
| Monoolein DOPS | - | Hydration Vortex mixing Centrifugation | 6.7 2.75 2.55 | L HII Im3m | Drug delivery (perspective) | [66] |
| Monoolein N-Oleoyl-glycine N-(2-aminoethyl)-oleamide | Doxorubicin | Melting Hydration Centrifuge mixing | 5.5 7.5 | Pn3m Pn3m | Drug delivery (perspective) | [69] |
| Monoolein Oleic acid Vaccenic acid Gondoic acid Erucic acid Nervonic acid | Pluronic F127 PBS | Hydration Ultrasonication | 4.9 7.0 | Fd3m HII | Drug delivery (perspective) | [70] |
| Monoolein “Lipid 3” | Methylene green zinc chloride double salt | Hydration Centrifugation | 2.5 3.0 5.0 7.0 | Pn3m Pn3m Pn3m Pn3m | Drug delivery (perspective) | [71] |
| Monoolein | Nicergoline Pluronic F108 | Ultrasonication | 3.3; 5.6; 5.9; 6.7 7.2 8.4 | Im3m Im3m Pn3m, Im3m Pn3m, HII | Drug delivery (perspective) | [72] |
| Monolinolein | “Outer membrane protein F” | Heating 45 °C Vortex mixing | 4.8 7.4 | Pn3m Pn3m | Drug delivery (perspective) | [73] |
| Monoolein Monolinolein | Bupivacaine Caprylic acid Capric acid | Heating 50 °C Hydration Heating 60 °C Vortex mixing Incubation at 37 °C (1–2 weeks) | 6.0 7.4 | Pn3m HII | Drug delivery (perspective) | [74] |
| Phytantriol | Pluronic F127 Decyl betainate chloride | Ultrasonication | 3.9; 5.5 7.4; 8.5 | Pn3m, L Im3m, HII | Oral drug delivery (perspective) | [75] |
| DOPE | DNA N,N-dimethyldodecyl- amine-N-oxide | Hydration Vortex mixing Freeze–thaw | 7.2 4.8 | HII, L, Pn3m HII, L | Genetic and drug delivery (perspective) | [76] |
| Monoolein | PP50 1 Pluronic F127 | Hydration Sonication Stabilization with surfactant | 7.5 5.5 | Im3m Im3m, swollen | Drug delivery (perspective) | [77] |
| Monoolein Phytantriol | Poloxamer P407 PDMAEMA-b- PLMA | Hydration Ultrasonication | 4.2 6.0 7.4 | Im3m, L Im3m, L Im3m, L | Drug delivery (perspective) | [78] |
| Monoolein | Aspartic acid-leucine peptide Poly-lysine FITC–dextran | Melting 65 °C Hydration | 3.0; 5.0; 7.0; 8.5 | Not identified | Drug delivery (perspective) | [79] |
| Monoolein | Modified alginate Modified silk fibroin FITC–dextran | Melting 60 °C Hydration | 3.0; 4.0; 4.5; 5.0; 7.0; 9.0 | Not identified | Drug delivery (perspective) | [80] |
| DMPC DMPE | N,N-dimethyl- dodecylamine- N-oxide Poly(acrylic acid) | Hydration Repeated heating 60 °C, vortex mixing, ice bath cooling | <2 3.8 6.8 9.8 | L (swollen) L (swollen + collap) L (collap + multiL) Im3m, L (collap) | Therapeutic agent (perspective) | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials 2020, 10, 963. https://doi.org/10.3390/nano10050963
Mertins O, Mathews PD, Angelova A. Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials. 2020; 10(5):963. https://doi.org/10.3390/nano10050963
Chicago/Turabian StyleMertins, Omar, Patrick D. Mathews, and Angelina Angelova. 2020. "Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications" Nanomaterials 10, no. 5: 963. https://doi.org/10.3390/nano10050963
APA StyleMertins, O., Mathews, P. D., & Angelova, A. (2020). Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials, 10(5), 963. https://doi.org/10.3390/nano10050963