Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomic Layer Deposition of ZnO on SiO2: In-Situ Thermogravimetry
2.2. Scale up of Atomic Layer Deposition of ZnO on SiO2
2.3. Characterization of Materials
3. Results
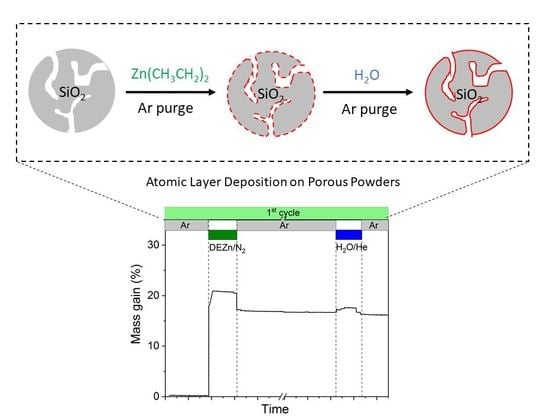
3.1. In-Situ Thermogravimetry
3.2. Scale-up of the ALD Process in a Fixed Bed Reactor
3.3. Characterization of ALD coated ZnO/SiO2 Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Djuriić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.K.; Baskoutas, S. Chemical sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MD, T. Doped zinc oxide nanostructures for photovoltaic solar cells application. In Zinc Oxide Based Nano Materials and Devices; IntechOpen: London, UK, 2019. [Google Scholar]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The applications of morphology controlled ZnO in catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Hussain, B.; Akhtar Raja, M.Y.; Lu, N.; Ferguson, I. Applications and synthesis of zinc oxide: An emerging wide bandgap material. In Proceedings of the 2013 High Capacity Optical Networks and Emerging/Enabling Technologies, Magosa, Cyprus, 11—13 December 2013; pp. 88–93. [Google Scholar]
- Shido, T.; Iwasawa, Y. The effect of coadsorbates in reverse water-gas shift reaction on ZnO, in relation to reactant-promoted reaction mechanism. J. Catal. 1993, 140, 575–584. [Google Scholar] [CrossRef]
- Nakamura, J.; Uchijima, T.; Kanai, Y.; Fujitani, T. The role of ZnO in Cu/ZnO methanol synthesis catalysts. Catal. Today 1996, 28, 223–230. [Google Scholar] [CrossRef]
- Schweitzer, N.M.; Hu, B.; Das, U.; Kim, H.; Greeley, J.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; Hock, A.S. Propylene hydrogenation and propane dehydrogenation by a single-site Zn2+ on silica catalyst. ACS Catal. 2014, 4, 1091–1098. [Google Scholar] [CrossRef]
- Camacho-Bunquin, J.; Aich, P.; Ferrandon, M.; “Bean” Getsoian, A.; Das, U.; Dogan, F.; Curtiss, L.A.; Miller, J.T.; Marshall, C.L.; Hock, A.S.; et al. Single-site zinc on silica catalysts for propylene hydrogenation and propane dehydrogenation: Synthesis and reactivity evaluation using an integrated atomic layer deposition-catalysis instrument. J. Catal. 2017, 345, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Naumann D’Alnoncourt, R.; Xia, X.; Strunk, J.; Löffler, E.; Hinrichsen, O.; Muhler, M. The influence of strongly reducing conditions on strong metal-support interactions in Cu/ZnO catalysts used for methanol synthesis. Phys. Chem. Chem. Phys. 2006, 8, 1525–1538. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Du, L.; Yao, H.; Wang, J.; Luo, G. Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Ind. Eng. Chem. Res. 2016, 55, 11943–11949. [Google Scholar] [CrossRef]
- Liao, F.; Han, X.; Zhang, Y.; Xu, C.; Chen, H. Hydrothermal synthesis of flower-like zinc oxide microstructures with large specific surface area. J. Mater. Sci. Mater. Electron. 2017, 28, 16855–16860. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Oneill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst design with atomic layer deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef] [Green Version]
- Elam, J.W.; Libera, J.A.; Huynh, T.H.; Feng, H.; Pellin, M.J. Atomic layer deposition of aluminum oxide in mesoporous silica gel. J. Phys. Chem. C 2010, 114, 17286–17292. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Kim, Y.D.; Park, S.; Kasinathan, P.; Hwang, Y.K.; Chang, J.-S.; Park, Y.-K. Preparation of ZnO/Al2O3 catalysts by using atomic layer deposition for plasma-assisted non-oxidative methane coupling. J. Korean Phys. Soc. 2016, 68, 1221–1227. [Google Scholar] [CrossRef]
- Mahurin, S.; Bao, L.; Yan, W.; Liang, C.; Dai, S. Atomic layer deposition of TiO2 on mesoporous silica. J. Non Cryst. Solids 2006, 352, 3280–3284. [Google Scholar] [CrossRef]
- Tammenmaa, M.; Koskinen, T.; Hiltunen, L.; Niinistö, L.; Leskelä, M. Zinc chalcogenide thin films grown by the atomic layer epitaxy technique using zinc acetate as source material. Thin Solid Films 1985, 124, 125–128. [Google Scholar] [CrossRef]
- Kaiya, K.; Yoshii, N.; Takahashi, N.; Nakamura, T. Atmospheric pressure atomic layer epitaxy of ZnO on a sapphire (0001) substrate by alternate reaction of ZnCl2 and O2. J. Mater. Sci. Lett. 2000, 19, 2089–2090. [Google Scholar] [CrossRef]
- Kobayashi, K.; Okudaira, S. Preparation of ZnO films on sapphire (0001) substrates by alternate supply of zinc acetate and H2O. Chem. Lett. 1997, 26, 511–512. [Google Scholar] [CrossRef]
- Lujala, V.; Skarp, J.; Tammenmaa, M.; Suntola, T. Atomic layer epitaxy growth of doped zinc oxide thin films from organometals. Appl. Surf. Sci. 1994, 82–83, 34–40. [Google Scholar] [CrossRef]
- Park, S.H.K.; Lee, Y.E. Controlling preferred orientation of ZnO thin films by atomic layer deposition. J. Mater. Sci. 2004, 39, 2195–2197. [Google Scholar] [CrossRef]
- Kim, C.R.; Lee, J.Y.; Shin, C.M.; Leem, J.Y.; Ryu, H.; Chang, J.H.; Lee, H.C.; Son, C.S.; Lee, W.J.; Jung, W.G.; et al. Effects of annealing temperature of buffer layer on structural and optical properties of ZnO thin film grown by atomic layer deposition. Solid State Commun. 2008, 148, 395–398. [Google Scholar] [CrossRef]
- Lin, P.Y.; Gong, J.R.; Li, P.C.; Lin, T.Y.; Lyu, D.Y.; Lin, D.Y.; Lin, H.J.; Li, T.C.; Chang, K.J.; Lin, W.J. Optical and structural characteristics of ZnO films grown on (0 0 0 1) sapphire substrates by ALD using DEZn and N2O. J. Cryst. Growth 2008, 310, 3024–3028. [Google Scholar] [CrossRef]
- Sanders, B.W.; Kitai, A. Zinc oxysulfide thin films grown by atomic layer deposition. Chem. Mater. 1992, 4, 1005–1011. [Google Scholar] [CrossRef]
- Butcher, K.S.; Chen, P.P.; Godlewski, M.; Szczerbakow, A.; Goldys, E.M.; Tansley, T.L.; Freitas, J.A. Recrystallization prospects for freestanding low-temperature GaN grown using ZnO buffer layers. J. Cryst. Growth 2002, 246, 237–243. [Google Scholar] [CrossRef]
- Kopalko, K.; Godlewski, M.; Guziewicz, E.; Łusakowska, E.; Paszkowicz, W.; Domagała, J.; Dynowska, E.; Szczerbakow, A.; Wójcik, A.; Phillips, M.R. Monocrystalline thin films of ZnSe and ZnO grown by atomic layer epitaxy. Vacuum 2004, 74, 269–272. [Google Scholar] [CrossRef]
- Yousfi, E.B.; Fouache, J.; Lincot, D. Study of atomic layer epitaxy of zinc oxide by in-situ quartz crystal microgravimetry. Appl. Surf. Sci. 2000, 153, 223–234. [Google Scholar] [CrossRef]
- Libera, J.A.; Elam, J.W.; Pellin, M.J. Conformal ZnO coatings on high surface area silica gel using atomic layer deposition. Thin Solid Films 2008, 516, 6158–6166. [Google Scholar] [CrossRef]
- Lei, Y.; Lee, S.; Low, K.B.; Marshall, C.L.; Elam, J.W. Combining electronic and geometric effects of ZnO-promoted Pt nanocatalysts for aqueous phase reforming of 1-propanol. ACS Catal. 2016, 6, 3457–3460. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.; Lu, J.; Feng, H. ZnO modified ZSM-5 and y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, W.Y.; Xiao, G.M. Atomic layer deposition of zinc oxide on HZSM-5 template and its methanol aromatization performance. Catal. Lett. 2015, 145, 860–867. [Google Scholar] [CrossRef]
- Onn, T.M.; Küngas, R.; Fornasiero, P.; Huang, K.; Gorte, R.J. Atomic layer deposition on porous materials: Problems with conventional approaches to catalyst and fuel cell electrode preparation. Inorganics 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Strempel, V.E.; Naumann D’Alnoncourt, R.; Driess, M.; Rosowski, F. Atomic layer deposition on porous powders with in situ gravimetric monitoring in a modular fixed bed reactor setup. Rev. Sci. Instrum. 2017, 88, 074102. [Google Scholar] [CrossRef] [PubMed]
- Strempel, V.; Knemeyer, K.; Naumann d’Alnoncourt, R.; Driess, M.; Rosowski, F. Investigating the trimethylaluminium/water ALD process on mesoporous silica by in situ gravimetric monitoring. Nanomaterials 2018, 8, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingale, P.; Guan, C.; Kraehnert, R.; Naumann d Alnoncourt, R.; Thomas, A.; Rosowski, F. Design of an active and stable catalyst for dry reforming of methane via molecular layer deposition. Catal. Today 2020. [Google Scholar] [CrossRef]
- Lugmair, C.G.; Fujdala, K.L.; Tilley, T.D. New tris(tert-butoxy)siloxy complexes of aluminum and their transformation to homogeneous aluminosilicate materials via low-temperature thermolytic pathways. Chem. Mater. 2002, 14, 888–898. [Google Scholar] [CrossRef]
- Sandoval, J.J.; Palma, P.; Álvarez, E.; Cámpora, J.; Rodríguez-Delgado, A. Mechanism of alkyl migration in diorganomagnesium 2,6-bis(imino)pyridine complexes: Formation of grignard-type complexes with square-planar Mg(II) centers. Organometallics 2016, 35, 3197–3204. [Google Scholar] [CrossRef] [Green Version]
- Weckman, T.; Laasonen, K. Atomic layer deposition of zinc oxide: Diethyl zinc reactions and surface saturation from first-principles. J. Phys. Chem. C 2016, 120, 21460–21471. [Google Scholar] [CrossRef] [Green Version]
- Weckman, T.; Laasonen, K. Atomic layer deposition of zinc oxide: Study on the water pulse reactions from first-principles. J. Phys. Chem. C 2018, 122, 7685–7694. [Google Scholar] [CrossRef]
- Worrell, J.H. Inorganic Chemistry: An Industrial and Environmental Perspective (Swaddle, T. W.). J. Chem. Educ. 1997, 74, 1399. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Ma, Z.; Wejinya, U.; Zou, M.; Liu, Y.; Zhou, H.; Meng, X. A revisit to atomic layer deposition of zinc oxide using diethylzinc and water as precursors. J. Mater. Sci. 2019, 54, 5236–5248. [Google Scholar] [CrossRef]






| Sample Name | ALD Cycles | Specific Surface Area (m²/g) | Average Pore Volume (cc/g) | ZnO Content (XRF) (wt. %) | Total Mass Gain from Balance (wt.%) |
|---|---|---|---|---|---|
| SiO2 | 0 | 505 | 0.75 | 0 | 0 |
| 1c-ZnO/SiO2 | 1 | 400 | 0.56 | 15.7 | 16 |
| 2c-ZnO/SiO2 | 2 | 289 | 0.43 | 31.8 | 32 |
| 3c-ZnO/SiO2 | 3 | 213 | 0.31 | 45.1 | 46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingale, P.; Knemeyer, K.; Piernavieja Hermida, M.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis. Nanomaterials 2020, 10, 981. https://doi.org/10.3390/nano10050981
Ingale P, Knemeyer K, Piernavieja Hermida M, Naumann d’Alnoncourt R, Thomas A, Rosowski F. Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis. Nanomaterials. 2020; 10(5):981. https://doi.org/10.3390/nano10050981
Chicago/Turabian StyleIngale, Piyush, Kristian Knemeyer, Mar Piernavieja Hermida, Raoul Naumann d’Alnoncourt, Arne Thomas, and Frank Rosowski. 2020. "Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis" Nanomaterials 10, no. 5: 981. https://doi.org/10.3390/nano10050981
APA StyleIngale, P., Knemeyer, K., Piernavieja Hermida, M., Naumann d’Alnoncourt, R., Thomas, A., & Rosowski, F. (2020). Atomic Layer Deposition of ZnO on Mesoporous Silica: Insights into Growth Behavior of ZnO via In-Situ Thermogravimetric Analysis. Nanomaterials, 10(5), 981. https://doi.org/10.3390/nano10050981