(Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Development of Nanostructured Fe3O4 Materials
3.2. Development of Nanocomposite (Ag)Pd-Fe3O4 Materials
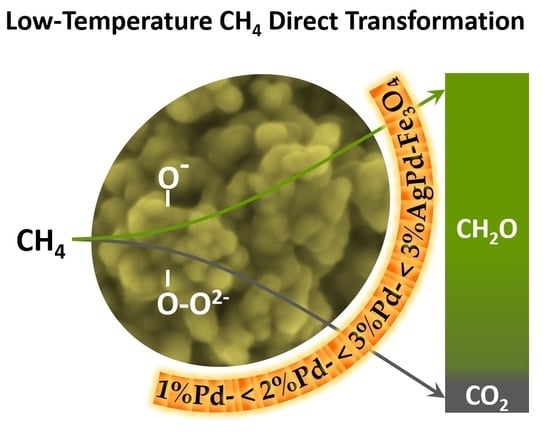
3.3. Catalytic Tests for Methane Partial Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hargreaves, J.S.J.; Hutchings, G.J.; Joyner, R.W. Control of product selectivity in the partial oxidation of methane. Nature 1990, 348, 428–429. [Google Scholar] [CrossRef]
- Zhao, G.; Drewery, M.; Mackie, J.; Oliver, T.; Kennedy, E.M.; Stockenhuber, M. The Catalyzed Conversion of Methane to Value-Added Products. Energy Technol. 2019, 1900665. [Google Scholar] [CrossRef]
- Fait, M.J.G.; Ricci, A.; Holena, M.; Rabeah, J.; Pohl, M.M.; Linke, D.; Kondratenko, E.V. Understanding trends in methane oxidation to formaldehyde: Statistical analysis of literature data and based hereon experiments. Catal. Sci. Technol. 2019, 9, 5111–5121. [Google Scholar] [CrossRef]
- Smith, M.R.; Ozkan, U.S. The Partial Oxidation of Methane to Formaldehyde: Role of Different Crystal Planes of MoO3. J. Catal. 1993, 141, 124–139. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Rekoske, J.E.; Dumesic, J.A.; Rudd, D.F.; Spencer, N.D.; Pereira, C.J. Simulation of methane partial oxidation over silica-supported MoO3 and V2O5. Aiche J. 1991, 37, 87–97. [Google Scholar] [CrossRef]
- Mac Giolla Coda, E.; Kennedy, M.; McMonagle, J.B.; Hodnett, B.K. Oxidation of methane to formaldehyde over supported molybdena catalysts at ambient pressure: Isolation of the selective oxidation product. Catal. Today 1990, 6, 559–566. [Google Scholar] [CrossRef]
- Smith, M.R.; Zhang, L.; Driscoll, S.A.; Ozkan, U.S. Effect of surface species on activity and selectivity of MoO3/SiO2 catalysts in partial oxidation of methane to formaldehyde. Catal. Lett. 1993, 19, 1–15. [Google Scholar] [CrossRef]
- de Vekki, A.V.; Marakaev, S.T. Catalytic partial oxidation of methane to formaldehyde. Russ. J. Appl. Chem. 2009, 82, 521–536. [Google Scholar] [CrossRef]
- Zhao, G.; Benhelal, E.; Adesina, A.; Kennedy, E.; Stockenhuber, M. Comparison of Direct, Selective Oxidation of Methane by N2O over Fe-ZSM-5, Fe-βeta, and Fe-FER Catalysts. J. Phys. Chem. C 2019, 123, 27436–27447. [Google Scholar] [CrossRef]
- Fajardo, C.A.G.; Niznansky, D.; N’Guyen, Y.; Courson, C.; Roger, A.-C. Methane selective oxidation to formaldehyde with Fe-catalysts supported on silica or incorporated into the support. Catal. Commun. 2008, 9, 864–869. [Google Scholar] [CrossRef]
- Kobayashi, T.; Guilhaume, N.; Miki, J.; Kitamura, N.; Haruta, M. Oxidation of methane to formaldehyde over FeSiO2 and Sn-W mixed oxides. Catal. Today 1996, 32, 171–175. [Google Scholar] [CrossRef]
- Brown, M.J.; Parkyns, N.D. Progress in the partial oxidation of methane to methanol and formaldehyde. Catal. Today 1991, 8, 305–335. [Google Scholar] [CrossRef]
- Parmaliana, A.; Sokolovskii, V.; Miceli, D.; Arena, F.; Giordano, N. Silica-Supported MoO3 and V2O5 Catalysts in Partial Oxidation of Methane to Formaldehyde. In Catalytic Selective Oxidation; American Chemical Society: Washington, DC, USA, 1993; Volume 523, pp. 43–57. [Google Scholar]
- He, J.; Li, Y.; An, D.; Zhang, Q.; Wang, Y. Selective oxidation of methane to formaldehyde by oxygen over silica-supported iron catalysts. J. Nat. Gas Chem. 2009, 18, 288–294. [Google Scholar] [CrossRef]
- Parmaliana, A.; Arena, F.; Frusteri, F.; Martínez-Arias, A.; Granados, M.; Fierro, J.L.G. Effect of Fe-addition on the catalytic activity of silicas in the partial oxidation of methane to formaldehyde. Appl. Catal. A Gen. 2002, 226, 163–174. [Google Scholar] [CrossRef]
- Arena, F.; Gatti, G.; Martra, G.; Coluccia, S.; Stievano, L.; Spadaro, L.; Famulari, P.; Parmaliana, A. Structure and reactivity in the selective oxidation of methane to formaldehyde of low-loaded FeOx /SiO2 catalysts. J. Catal. 2005, 231, 365–380. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Yang, L.; Wang, X.; Zhang, Q. Iron-containing heterogeneous catalysts for partial oxidation of methane and epoxidation of propylene. Catal. Today 2006, 117, 156–162. [Google Scholar] [CrossRef]
- Shimura, K.; Fujitani, T. Effects of promoters on the performance of a VOx/SiO2 catalyst for the oxidation of methane to formaldehyde. Appl. Catal. A Gen. 2019, 577, 44–51. [Google Scholar] [CrossRef]
- Aoki, K.; Ohmae, M.; Nanba, T.; Takeishi, K.; Azuma, N.; Ueno, A.; Ohfune, H.; Hayashi, H.; Udagawa, Y. Direct conversion of methane into methanol over MoO3/SiO2 catalyst in an excess amount of water vapor. Catal. Today 1998, 45, 29–33. [Google Scholar] [CrossRef]
- Koranne, M.M.; Goodwin, J.G.; Marcelin, G. Carbon pathways for the partial oxidation of methane. J. Phys. Chem. 1993, 97, 673–678. [Google Scholar] [CrossRef]
- Yamada, Y.; Ichihashi, Y.; Ando, H.; Ueda, A.; Shioyama, H.; Kobayashi, T. Simple Preparation Method of Isolated Iron (III) Species on Silica Surface. Chem. Lett. 2003, 32, 208–209. [Google Scholar] [CrossRef]
- Kobayashi, T. Selective oxidation of light alkanes to aldehydes over silica catalysts supporting mononuclear active sites - Acrolein formation from ethane. Catal. Today 2001, 71, 69–76. [Google Scholar] [CrossRef]
- Kado, S.; Urasaki, K.; Sekine, Y.; Fujimoto, K. Direct conversion of methane to acetylene or syngas at room temperature using non-equilibrium pulsed discharge. Fuel 2003, 82, 1377–1385. [Google Scholar] [CrossRef]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by using Copper-Promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Dietl, N.; Kretschmer, R.; Ma, J.-B.; Weiske, T.; Schlangen, M.; Schwarz, H. Direct Conversion of Methane into Formaldehyde Mediated by [Al2O3]·+ at Room Temperature. Angew. Chem. Int. Ed. 2012, 51, 3703–3707. [Google Scholar] [CrossRef] [PubMed]
- Grunwaldt, J.-D.; van Vegten, N.; Baiker, A. Insight into the structure of supported palladium catalysts during the total oxidation of methane. Chem. Commun. 2007, 4635–4637. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhu, Y.X.; Wu, N.Z.; Xie, Y.C.; Murwani, I.; Kemnitz, E. Total oxidation of methane at low temperature over Pd/TiO2/Al2O3: Effects of the support and residual chlorine ions. Appl. Catal. B Environ. 2004, 50, 59–66. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Kröcher, O.; van Bokhoven, J.A. Design of Stable Palladium-Based Zeolite Catalysts for Complete Methane Oxidation by Postsynthesis Zeolite Modification. Acs Catal. 2019, 9, 2303–2312. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Catalytic Deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Khan, N.A.; Uhl, A.; Shaikhutdinov, S.; Freund, H.J. Alumina supported model Pd–Ag catalysts: A combined STM, XPS, TPD and IRAS study. Surf. Sci. 2006, 600, 1849–1853. [Google Scholar] [CrossRef]
- González, S.; Neyman, K.M.; Shaikhutdinov, S.; Freund, H.-J.; Illas, F. On the Promoting Role of Ag in Selective Hydrogenation Reactions over Pd−Ag Bimetallic Catalysts: A Theoretical Study. J. Phys. Chem. C 2007, 111, 6852–6856. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Li, Y.-S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.; Vasile, E.; Predescu, A. Properties of magnetic iron oxides used as materials for wastewater treatment. J. Phys. Conf. Ser. 2011, 304, 012022. [Google Scholar] [CrossRef]
- van Oorschot, I.H.M.; Dekkers, M.J. Dissolution behaviour of fine-grained magnetite and maghemite in the citrate–bicarbonate–dithionite extraction method. Earth Planet. Sci. Lett. 1999, 167, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.Y.; Kwak, S.-Y. Assembly of magnetite nanocrystals into spherical mesoporous aggregates with a 3-D wormhole-like pore structure. J. Mater. Chem. 2010, 20, 8320–8328. [Google Scholar] [CrossRef]
- Pecharromán, C.; González-Carreño, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, γ-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Sun, D.; Li, P.; Yang, B.; Xu, Y.; Huang, J.; Li, Q. Monodisperse AgPd alloy nanoparticles as a highly active catalyst towards the methanolysis of ammonia borane for hydrogen generation. Rsc Adv. 2016, 6, 105940–105947. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, F.; Guo, L.; Jin, T.; Liu, H.; Wang, X.; Gong, X.; Liu, Y. Nanoalloying effects on the catalytic activity of the formate oxidation reaction over AgPd and AgCuPd aerogels. J. Mater. Chem. A 2019, 7, 16122–16135. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Huang, J.; Du, M.; Yang, F.; Chen, H.; Hong, Y.; Li, Q. Plant-Mediated Synthesis of Ag–Pd Alloy Nanoparticles and Their Application as Catalyst toward Selective Hydrogenation. ACS Sustain. Chem. Eng. 2014, 2, 1212–1218. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Jiang, X. Plant-mediated synthesis of AgPd/γ-Al2O3 catalysts for selective hydrogenation of 1,3-butadiene at low temperature. New J. Chem. 2019, 43, 13891–13898. [Google Scholar] [CrossRef]
- Bish, D.L.; Post, J.E. Modern powder diffraction. In Reviews in Mineralogy & Geochemistry; America, I.S.M.S.O., Ed.; De Gruyter: Washington, DC, USA, 1989; Volume 20. [Google Scholar]
- Veisi, H.; Najafi, S.; Hemmati, S. Pd(II)/Pd(0) anchored to magnetic nanoparticles (Fe3O4) modified with biguanidine-chitosan polymer as a novel nanocatalyst for Suzuki-Miyaura coupling reactions. Int. J. Biol. Macromol. 2018, 113, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bristy, S.S.; Rahman, M.A.; Tauer, K.; Minami, H.; Ahmad, H. Preparation and characterization of magnetic γ-Al2O3 ceramic nanocomposite particles with variable Fe3O4 content and modification with epoxide functional polymer. Ceram. Int. 2018, 44, 3951–3959. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P.; Wagner, F.E.; Berensmeier, S. Oxidation of magnetite nanoparticles: Impact on surface and crystal properties. CrystEngComm 2017, 19, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Boucherit, N.; Hugot-Le Goff, A.; Joiret, S. Raman studies of corrosion films grown on Fe and Fe-6Mo in pitting conditions. Corros. Sci. 1991, 32, 497–507. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Araujo, J.F.D.F.; Tahir; Arsalani, S.; Freire, F.L.; Mariotto, G.; Cremona, M.; Mendoza, L.A.F.; Luz-Lima, C.; Zaman, Q.; Del Rosso, T.; et al. Novel scanning magnetic microscopy method for the characterization of magnetic nanoparticles. J. Magn. Magn. Mater. 2020, 499, 166300. [Google Scholar] [CrossRef]
- Hu, L.; Hach, D.; Chaumont, D.; Brachais, C.H.; Couvercelle, J.P. One step grafting of monomethoxy poly(ethylene glycol) during synthesis of maghemite nanoparticles in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 1–7. [Google Scholar] [CrossRef]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core-shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef] [Green Version]
- Daou, T.J.; Grenèche, J.M.; Pourroy, G.; Buathong, S.; Derory, A.; Ulhaq-Bouillet, C.; Donnio, B.; Guillon, D.; Begin-Colin, S. Coupling Agent Effect on Magnetic Properties of Functionalized Magnetite-Based Nanoparticles. Chem. Mater. 2008, 20, 5869–5875. [Google Scholar] [CrossRef]
- Rečnik, A.; Nyirő-Kósa, I.; Dódony, I.; Pósfai, M. Growth defects and epitaxy in Fe3O4 and γ-Fe2O3 nanocrystals. CrystEngComm 2013, 15, 7539–7547. [Google Scholar] [CrossRef]
- Oblonsky, L.J.; Devine, T.M. A surface-enhanced Raman spectroscopic study of the passive films formed in borate buffer on iron, nickel, chromium and stainless steel. Corros. Sci. 1995, 37, 17–41. [Google Scholar] [CrossRef]
- Kozlova, A.P.; Sugiyama, S.; Kozlov, A.I.; Asakura, K.; Iwasawa, Y. Iron-Oxide Supported Gold Catalysts Derived from Gold-Phosphine Complex Au(PPh3)(NO3): State and Structure of the Support. J. Catal. 1998, 176, 426–438. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F., Jr.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Kubo, K.; Sato, N. Raman Spectroscopy of Thin Corrosion Films on Iron at 100 to 150 ≡C in Air. Corrosion 1986, 42, 476–481. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. Acs Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films and powders produced by sol-gel syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Marshall, C.P.; Dufresne, W.J.B.; Rufledt, C.J. Polarized Raman spectra of hematite and assignment of external modes. J. Raman Spectrosc. 2020. [Google Scholar] [CrossRef]
- El Mendili, P.Y.; Grasset, F.; Randrianantoandro, N.; Nerambourg, N.; Greneche, J.-M.; Bardeau, J.F. Improvement of Thermal Stability of Maghemite Nanoparticles Coated With Oleic Acid and Oleylamine Molecules: Investigations Under Laser Irradiation. J. Phys. Chem. C 2015, 119, 10662–10668. [Google Scholar] [CrossRef]
- Singh, R.; Misra, V.; Singh, R. Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. J. Nanopart. Res. 2011, 13, 4063–4073. [Google Scholar] [CrossRef]
- Waser, J.; Levy, H.A.; Peterson, S.W. The structure of PdO. Acta Crystallogr. 1953, 6, 661–663. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd° oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. PCCP 2011, 13, 4607–4613. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Hayashi, Y.; Takizawa, H. Synthesis of Palladium Nanoparticles and Palladium/Spherical Carbon Composite Particles in the Solid-Liquid System of Palladium Oxide-Alcohol by Microwave Irradiation. Mater. Trans. 2011, 52, 1048–1052. [Google Scholar] [CrossRef] [Green Version]
- Formaldehyde Health and Safety Guide; IPCS International Programme on Chemical Safety Health and Safety Guide No. 57; World Health Organization: Geneva, Switzerland, 1991.
- Yang, X.; Jung, K.D.; Cho, S.H.; Joo, O.S.; Uhm, S.J.; Han, S.H. Low-temperature oxidation of methane to form formaldehyde: Role of Fe and Mo on Fe–Mo/SiO2 catalysts, and their synergistic effects. Catal. Lett. 2000, 64, 185–190. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kudrik, E.V.; Alvarez, L.X.; Afanasiev, P.; Millet, J.M.M.; Bouchu, D. Oxidation of methane and ethylene in water at ambient conditions. Catal. Today 2010, 157, 149–154. [Google Scholar] [CrossRef]
- Merkx, M.; Kopp, D.A.; Sazinsky, M.H.; Blazyk, J.L.; Müller, J.; Lippard, S.J. Dioxygen Activation and Methane Hydroxylation by Soluble Methane Monooxygenase: A Tale of Two Irons and Three Proteins. Angew. Chem. Int. Ed. 2001, 40, 2782–2807. [Google Scholar] [CrossRef]
- Yamada, Y.; Ueda, A.; Shioyama, H.; Kobayashi, T. High throughput experiments on methane partial oxidation using molecular oxygen over silica doped with various elements. Appl. Catal. A Gen. 2003, 254, 45–58. [Google Scholar] [CrossRef]
- Pitchai, R.; Klier, K. Partial Oxidation of Methane. Catal. Rev. 1986, 28, 13–88. [Google Scholar] [CrossRef]
- Milone, C.; Ingoglia, R.; Schipilliti, L.; Crisafulli, C.; Neri, G.; Galvagno, S. Selective hydrogenation of α,β-unsaturated ketone to α,β-unsaturated alcohol on gold-supported iron oxide catalysts: Role of the support. J. Catal. 2005, 236, 80–90. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Zhao, K.; He, D. Preparation of Palladium Supported on Ferric Oxide Nano-catalysts for Carbon Monoxide Oxidation in Low Temperature. Nano-Micro Lett. 2014, 6, 233–241. [Google Scholar] [CrossRef]
- Mitsui, T.; Rose, M.K.; Fomin, E.; Ogletree, D.F.; Salmeron, M. Dissociative hydrogen adsorption on palladium requires aggregates of three or more vacancies. Nature 2003, 422, 705–707. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.-G.; Wu, J.-P.; Cheng, H.-S. Hydrogen adsorption and desorption on the Pt and Pd subnano clusters—A review. Front. Phys. China 2009, 4, 356–366. [Google Scholar] [CrossRef]









| Fe3+/Fe2+ | Temperature (°C) | SBET (m2 g−1) 1 |
|---|---|---|
| 2 | 110 | 77.0 |
| 2 | 95 | 91.3 |
| 2 | 80 | 96.9 |
| 4 | 110 | 125.7 |
| 4 | 95 | 135.8 |
| 4 | 80 | 137.6 |
| Catalyst | Metal Load 1 (wt.%) | Pd Load 1 (wt.%) | Pd:Ag 2 | Pd:Ag (SA-EDS) | Diam. Metal NPs 4 (nm) |
|---|---|---|---|---|---|
| 1% Pd-Fe3O4 | 1 | 1 | 1:0 | - | 3.3 ± 1.2 |
| 2% Pd-Fe3O4 | 2 | 2 | 1:0 | - | 3.4 ± 1.1 |
| 3% Pd-Fe3O4 | 3 | 3 | 1:0 | - | 4.4 ± 1.6 |
| 3% AgPd-Fe3O4 | 3 | 2.4 | 0.8:0.2 | 0.8:0.2 3 | 4.3 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Navarro, B.; Sanchis, R.; Asedegbega-Nieto, E.; Solsona, B.; Ivars-Barceló, F. (Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature. Nanomaterials 2020, 10, 988. https://doi.org/10.3390/nano10050988
Martínez-Navarro B, Sanchis R, Asedegbega-Nieto E, Solsona B, Ivars-Barceló F. (Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature. Nanomaterials. 2020; 10(5):988. https://doi.org/10.3390/nano10050988
Chicago/Turabian StyleMartínez-Navarro, Blanca, Ruth Sanchis, Esther Asedegbega-Nieto, Benjamín Solsona, and Francisco Ivars-Barceló. 2020. "(Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature" Nanomaterials 10, no. 5: 988. https://doi.org/10.3390/nano10050988
APA StyleMartínez-Navarro, B., Sanchis, R., Asedegbega-Nieto, E., Solsona, B., & Ivars-Barceló, F. (2020). (Ag)Pd-Fe3O4 Nanocomposites as Novel Catalysts for Methane Partial Oxidation at Low Temperature. Nanomaterials, 10(5), 988. https://doi.org/10.3390/nano10050988