Neutrophils as Main Players of Immune Response towards Nondegradable Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Potential Mechanisms for “Inactivation” of Bio-Resistant Nanoparticles
3.2. Effect of Nano/Microparticle Size on Its Interaction with Cells or Cell Membranes
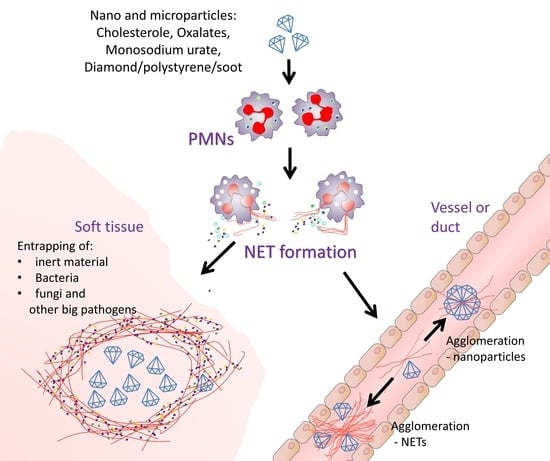
3.3. Membrane Leakage Can Kill Cells or Induce NETs Formation
3.4. Dietary-Born Nano/Microparticle and NETs Formation
3.4.1. Monosodium Urate Nanocrystals
3.4.2. Cholesterol Nanocrystals
3.4.3. Fructose
3.5. Consequences of Nano/Microparticle Aggregation in Tissues and Ducts/Vessels
3.5.1. Beneficial Role of Nano/Microparticle Aggregation
3.5.2. Detrimental Effect of Nanoparticle-NET Aggregates
4. Discussion
Supplementary Materials
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Anders, H.-J.; Mulay, S.R.; Herrmann, M.; Bilyy, R.; Gabibov, G.G. Editorial on the research topic: Nano-and Microparticle-Induced Cell Death, Inflammation and Immune Responses. Front. Immunol. 2019, 10, 844. [Google Scholar]
- Tomin, A.; Dumych, T.; Kril, I.; Antonyuk, V.; Chopyak, V.; Munoz, L.; Stoika, R.; Herrmann, M.; Bilyy, R. Magnetic separation of apoptotic cells with lectin-conjugated microparticles: Magnetische Abtrennung apoptotischer Zellen mit Lektin-konjugierten Mikropartikeln. Materwiss. Werksttech. 2016, 47, 189–192. [Google Scholar] [CrossRef]
- Bila, G.; Schneider, M.; Peshkova, S.; Krajnik, B.; Besh, L.; Lutsyk, A.; Matsyura, O.; Bilyy, R. Novel approach for discrimination of eosinophilic granulocytes and evaluation of their surface receptors in a multicolor fluorescent histological assessment. Ukr. Biochem. J. 2020, 92, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Grüneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity 2019, 51, 443–450.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, Y.M.; Sedgwick, A.D.; Chea, E.P.; Willoughby, D.A. Mast cells in newly formed lining tissue during acute inflammation: A six day air pouch model in the mouse. Ann. Rheum. Dis. 1986, 45, 873–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppkes, M.; Maueröder, C.; Hirth, S.; Nowecki, S.; Günther, C.; Billmeier, U.; Paulus, S.; Biermann, M.; Munoz, L.E.E.; Hoffmann, M.; et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun. 2016, 7, 10973. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxidants Redox Signal. 2013, 18, 642–660. [Google Scholar] [CrossRef]
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 1. [Google Scholar] [CrossRef]
- Bilyy, R.; Fedorov, V.; Vovk, V.; Leppkes, M.; Dumych, T.; Chopyak, V.; Schett, G.; Herrmann, M. Neutrophil Extracellular Traps Form a Barrier between Necrotic and Viable Areas in Acute Abdominal Inflammation. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.; Radic, M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients with COVID-19. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Jiménez-Alcázar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renné, C.; Renné, T.; et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017, 358, 1202–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, L.E.; Bilyy, R.; Biermann, M.H.C.; Kienhöfer, D.; Maueröder, C.; Hahn, J.; Brauner, J.M.; Weidner, D.; Chen, J.; Scharin-Mehlmann, M.; et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E5856–E5865. [Google Scholar] [CrossRef] [Green Version]
- Agudo-Canalejo, J.; Lipowsky, R. Critical Particle Sizes for the Engulfment of Nanoparticles by Membranes and Vesicles with Bilayer Asymmetry. ACS Nano 2015, 9, 3704–3720. [Google Scholar] [CrossRef]
- Prylutska, S.; Bilyy, R.; Overchuk, M.; Bychko, A.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.G.; Ritter, U. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J. Biomed. Nanotechnol. 2012, 8, 522–527. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Desai, J.; Foresto-Neto, O.; Honarpisheh, M.; Steiger, S.; Nakazawa, D.; Popper, B.; Buhl, E.M.M.; Boor, P.; Mulay, S.R.R.; Anders, H.-J.J.J. Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Prylutska, S.; Bilyy, R.; Schkandina, T.; Bychko, A.; Cherepanov, V.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Scharff, P.; et al. Effect of iron-doped multi-walled carbon nanotubes on lipid model and cellular plasma membranes. Mater. Sci. Eng. C 2012, 32, 1486–1489. [Google Scholar] [CrossRef]
- Prylutska, S.; Bilyy, R.; Shkandina, T.; Rotko, D.; Bychko, A.; Cherepanov, V.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.; et al. Comparative study of membranotropic action of single- and multi-walled carbon nanotubes. J. Biosci. Bioeng. 2013, 115, 674–679. [Google Scholar] [CrossRef]
- Paryzhak, S.Y.; Dumych, T.I.; Peshkova, S.M.; Bila, E.E.; Lutsyk, A.D.; Barras, A.; Boukherroub, R.; Szunerits, S.; Bilyy, R.O. Interaction of 4 allotropic modifications of carbon nanoparticles with living tissues. Ukr. Biochem. J. 2019, 91, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Bila, G.; Peshkova, S.; Vovk, V.; Borysov, A.; Borisova, T.; Bilyy, R. Soot-derived carbon nanoparticles are encapsulated in the lungs upon inhalation. In Proceedings of the RECOOP Bridges in Life Science Annual Conference; Vari, S.G., Ed.; RECOOP HST Assosiation: LA, CA, USA, 2020; p. 15. [Google Scholar]
- Mulay, S.R.; Honarpisheh, M.M.; Foresto-Neto, O.; Shi, C.; Desai, J.; Zhao, Z.B.; Marschner, J.A.; Popper, B.; Buhl, E.M.; Boor, P.; et al. Mitochondria permeability transition versus necroptosis in oxalate-induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Schauer, C.; Janko, C.; Munoz, L.E.L.E.; Zhao, Y.Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Tausche, A.K.; Unger, S.; Richter, K.; Wunderlich, C.; Grässler, J.; Roch, B.; Schröder, H.E. Hyperuricemia and gout: Diagnosis and therapy. Internist (Berl.) 2006, 47, 509–520, quiz 521. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Schorn, C.; Strysio, M.; Janko, C.; Munoz, L.E.; Schett, G.; Herrmann, M. The uptake by blood-borne phagocytes of monosodium urate is dependent on heat-labile serum factor(s) and divalent cations. Autoimmunity 2010, 43, 236–238. [Google Scholar] [CrossRef]
- Schorn, C.; Frey, B.; Lauber, K.; Janko, C.; Strysio, M.; Keppeler, H.; Gaipl, U.S.; Voll, R.E.; Springer, E.; Munoz, L.E.; et al. Sodium overload and water influx activate the NALP3 inflammasome. J. Biol. Chem. 2011, 286, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Schett, G.; Schauer, C.; Hoffmann, M.; Herrmann, M. Why does the gout attack stop? A roadmap for the immune pathogenesis of gout. RMD Open 2015, 1, e000046. [Google Scholar] [CrossRef] [Green Version]
- Mulay, S.R.; Desai, J.; Kumar, S.V.S.V.; Eberhard, J.N.; Thomasova, D.; Romoli, S.; Grigorescu, M.; Kulkarni, O.P.O.P.; Popper, B.; Vielhauer, V.; et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat. Commun. 2016, 7, 10274. [Google Scholar] [CrossRef]
- Bilyy, R.; Lutsyk, A. A brief account of Julius Planer’s life and research. Condens. Matter Phys. 2010, 13, 370031–370033. [Google Scholar] [CrossRef]
- Admirand, W.H.; Small, D.M. The physicochemical basis of cholesterol gallstone formation in man. J. Clin. Invest. 1968, 47, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Bila, G.; Peshkova, S.; Dumych, T.; Bilyy, R. Natural cholesterol nanocrystals in gall material and their interaction with neutrophilic granulocytes. In Proceedings of the PhoBiA Annual Nanophotonics International Conference; Wroclaw University of Technology: Wroclaw, Poland, 2019; p. 62. [Google Scholar]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.W.; Dumke, K.A.; Goran, M.I. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition 2014, 30, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raivio, K.O.; Kekomäki, M.; Mäenpää, P. Fructose and purine metabolism. Acta Med. Scand. 1972, 192, 111–114. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Bila, G.; Vishchur, O.; Bilyy, R. High content of dietary fructose stimulates the formation of neutrophil extracellular traps in the biliary system. Exp. Clin. Physiol. Biochem. 2020, 89. [Google Scholar] [CrossRef]
- Paryzhak, S.; Dumych, T.; Mahorivska, I.; Boichuk, M.; Bila, G.; Peshkova, S.; Nehrych, T.; Bilyy, R. Neutrophil-released enzymes can influence composition of circulating immune complexes in multiple sclerosis. Autoimmunity 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Biermann, M.H.C.; Podolska, M.J.; Knopf, J.; Reinwald, C.; Weidner, D.; Maueröder, C.; Hahn, J.; Kienhöfer, D.; Barras, A.; Boukherroub, R.; et al. Oxidative burst-dependent NETosis is implicated in the resolution of necrosis-associated sterile inflammation. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Stephen, J.; Scales, H.E.; Benson, R.A.; Erben, D.; Garside, P.; Brewer, J.M. Neutrophil swarming and extracellular trap formation play a significant role in Alum adjuvant activity. NPJ Vaccines 2017, 2, 1. [Google Scholar] [CrossRef]
- Bilyy, R.; Paryzhak, S.; Turcheniuk, K.; Dumych, T.; Barras, A.; Boukherroub, R.; Wang, F.; Yushin, G.; Szunerits, S. Aluminum oxide nanowires as safe and effective adjuvants for next-generation vaccines. Mater. Today 2019, 22, 58–66. [Google Scholar] [CrossRef]
- Boeltz, S.; Muñoz, L.E.; Fuchs, T.A.; Herrmann, M. Neutrophil Extracellular Traps Open the Pandora’s Box in Severe Malaria. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarin-Bidóia, D.; Machado, R.R.B.; Nakamura, C.V.; Anders, H.J.; Steiger, S.; Silva Lautenschlager, S. de O. Serum Proteins Alter the Ability of Neutrophils to Phagocytose Malaria Related Hemozoin Crystals. Microsc. Microanal. 2020, 26, 153–154. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilyy, R.; Bila, G.; Vishchur, O.; Vovk, V.; Herrmann, M. Neutrophils as Main Players of Immune Response towards Nondegradable Nanoparticles. Nanomaterials 2020, 10, 1273. https://doi.org/10.3390/nano10071273
Bilyy R, Bila G, Vishchur O, Vovk V, Herrmann M. Neutrophils as Main Players of Immune Response towards Nondegradable Nanoparticles. Nanomaterials. 2020; 10(7):1273. https://doi.org/10.3390/nano10071273
Chicago/Turabian StyleBilyy, Rostyslav, Galyna Bila, Oleg Vishchur, Volodymyr Vovk, and Martin Herrmann. 2020. "Neutrophils as Main Players of Immune Response towards Nondegradable Nanoparticles" Nanomaterials 10, no. 7: 1273. https://doi.org/10.3390/nano10071273
APA StyleBilyy, R., Bila, G., Vishchur, O., Vovk, V., & Herrmann, M. (2020). Neutrophils as Main Players of Immune Response towards Nondegradable Nanoparticles. Nanomaterials, 10(7), 1273. https://doi.org/10.3390/nano10071273