Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System
Abstract
:1. Introduction
2. Materials, Methods, and Preparation
2.1. Materials
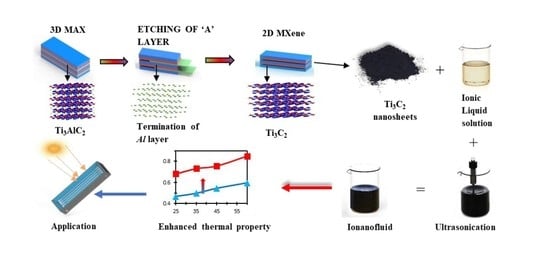
2.2. Synthesis of MXene (Ti3C2)
2.3. Preparation of Ionanofluid
2.4. Characterization
2.5. Thermophysical Properties Measurement
2.5.1. Thermal Conductivity
2.5.2. Specific Heat Capacity
2.5.3. Measurement of Viscosity
2.5.4. Measurement of Density
2.5.5. Measurement of Thermal Stability
2.5.6. Measurement of Zeta Potential
2.6. Physical Model of PV/T System
2.7. Numerical Modeling of PV/T Solar System
2.8. Boundary Conditions
2.9. Meshing and Grid Independence
3. Results and Discussions
3.1. Morphology and Characterization
3.2. Thermophysical Properties
3.2.1. Thermal Conductivity
3.2.2. Specific Heat
3.2.3. Viscosity
3.2.4. Density
3.2.5. Thermal Stability
3.3. Validation of Numerical Model
3.4. Performance of Solar PV/T System
4. Conclusions
- The 2D MXene were successfully synthesized from 3D MAX phase and SEM analyses were performed to inspect the morphology of MXene before formulating ionanofluids.
- The formulated ionanofluids showed good stability without adding any surfactants or chemical treatment. Optical property measurement also showed a significant improvement in absorbance (UV–vis analysis) capability which may be considered a promising aspect of solar energy storage systems. The FTIR analyses also showed that the MXene particles were well dispersed into the solution and they were chemically stable.
- Superior results were also obtained for thermophysical properties as the thermal conductivity enhancements are significant at each concentration of MXene; however, a maximum of 47% enhancement is noticed at 0.2 wt %. In addition, thermal conductivity increases substantially as the temperature rises from 20 °C to 60 °C.
- Interestingly, viscosity is found to be decreased by adding MXene nanosheets which might be attributed to their self-lubricating property. Specific heat increases with both increasing temperature and concentration while density is found to increase with concentration but decrease as temperature increases. TGA analysis also confirms that no significant decomposition occurs up to 60 °C within the samples.
- A simulation-based study has been conducted with IL+ water/MXene in a PV/T system along with two other nanofluids (water/alumina and palm oil/MXene) to assess the performance. IL+ water/MXene nanofluid with 20 wt % concentrations exhibits highest electrical efficiency, overall thermal efficiency, and heat transfer coefficient in comparison with water, Al3O3/water, and palm oil/MXene. Thermal efficiency of the considered PV/T system increases from 12.2 to 13.95% as flow rate increases from 0.01 to 0.07 kg/s with IL+ water/MXene. Moreover, thermal efficiency is also increased by 81.15% while, heat transfer coefficient is also increased by 12.6% and 2% for IL+ water/MXene compared to water/alumina and palm oil/MXene.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Nomenclature | σ | Stefan Boltzmann Constant, W/(m2.K4) | |
| Ac | area of collector (m2) | Subscripts | |
| cp | Specific heat (J/K) | amb | ambient |
| FF | field factor | el | electrical |
| G | solar radiation intensity (W/m2) | bf | base fluid |
| H | convective heat transfer coefficient (W/m2. K) | in | inlet |
| Isc | short circuit current (A) | out | outlet |
| knf | thermal conductivity of nanofluid (W/m.K) | s | solid particle |
| kbf | thermal conductivity of base fluid (W/m.K) | th | thermal |
| ks | thermal conductivity of nanoparticle (W/m.K) | nf | Nanofluids |
| Q՛conv | heat transfer due to convection (W) | Abbreviations | |
| Pth | thermal power output (W) | FTIR | Fourier-transform infrared spectroscopy |
| Pel | electrical power output (W) | NMR | nuclear magnetic resonance |
| Q՛rad | heat loss due to radiation (W) | IC | ion chromatographic |
| T | temperature (K) | KF | Karl Fischer |
| Voc | open circuit voltage (V) | IL | ionic liquid |
| Greeks | HPLC | high performance liquid chromatography | |
| TGA | thermogravimetric analysis | ||
| ζ | zeta potential, mV | SEM | scanning electron microscopy |
| Φ | nanoparticle weight fraction | PV/T | photovoltaic thermal |
| ρ | density, kg/m3 | SEM | scanning electron microscopy |
| η | efficiency | UV–vis | ultraviolet–visible spectroscopy |
| ε | emissivity | UDF | user defined function |
References
- IEA. World Energy Outlook 2019. 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 26 February 2020).
- Krishna, Y.; Faizal, M.; Saidur, R.; Ng, K.C.; Aslfattahi, N. State-of-the-art heat transfer fluids for parabolic trough collector. Int. J. Heat Mass Transf. 2020, 152, 119541. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Sidik, N.A.C.; Sabri, M.F.M.; Zahir, M.H. Experimental Assessment of a Novel Eutectic Binary Molten Salt-based Hexagonal Boron Nitride Nanocomposite as a Promising PCM with Enhanced Specific Heat Capacity. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 73–85. [Google Scholar] [CrossRef]
- Moghimi Ardekani, M. Optical Thermal and Economic Optimisation of a Linear Fresnel Collector; University of Pretoria: Pretoria, South Africa, 2017. [Google Scholar]
- Yang, M.; Wang, S.; Zhu, Y.; Taylor, R.A.; Moghimi, M.; Wang, Y.J.E. Thermal Stability and Performance Testing of Oil-based CuO Nanofluids for Solar Thermal Applications. Energies 2020, 13, 876. [Google Scholar] [CrossRef] [Green Version]
- Thirugnanasambandam, M.; Iniyan, S.; Goic, R. A review of solar thermal technologies. Renew. Sustain. Energy Rev. 2010, 14, 312–322. [Google Scholar] [CrossRef]
- Borode, A.; Ahmed, N.; Olubambi, P. A review of solar collectors using carbon-based nanofluids. J. Clean. Prod. 2019, 241, 118311. [Google Scholar] [CrossRef]
- Hjerrild, N.E.; Mesgari, S.; Crisostomo, F.; Scott, J.A.; Amal, R.; Taylor, R.A. Hybrid PV/T enhancement using selectively absorbing Ag–SiO2/carbon nanofluids. Sol. Energy Mater. Sol. Cells 2016, 147, 281–287. [Google Scholar] [CrossRef]
- Aguilar, T.; Sani, E.; Mercatelli, L.; Carrillo-Berdugo, I.; Torres, E.; Navas, J. Exfoliated graphene oxide-based nanofluids with enhanced thermal and optical properties for solar collectors in concentrating solar power. J. Mol. Liq. 2020, 306, 112862. [Google Scholar] [CrossRef]
- Sadeghzadeh, M.; Maddah, H.; Ahmadi, M.H.; Khadang, A.; Ghazvini, M.; Mosavi, A.; Nabipour, N. Prediction of Thermo-Physical Properties of TiO2-Al2O3/Water Nanoparticles by Using Artificial Neural Network. Nanomaterials 2020, 10, 697. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazik, A.; Tan, K.; Aslfattahi, N.; Arifutzzaman, A.; Saidur, R.; Al-Sulaiman, F.J.S.E. Optical, stability and energy performance of water-based MXene nanofluids in hybrid PV/thermal solar systems. Sol. Energy 2020, 204, 32–47. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 5282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zeng, G.; Lei, X. The stability, optical properties and solar-thermal conversion performance of SiC-MWCNTs hybrid nanofluids for the direct absorption solar collector (DASC) application. Sol. Energy Mater. Sol. Cells 2019, 206, 110323. [Google Scholar] [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. SiO2-ternary carbonate nanofluids prepared by mechanical mixing at high temperature: Enhanced specific heat capacity and thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 203, 110193. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Samylingam, L.; Abdelrazik, A.; Arifutzzaman, A.; Saidur, R.J.S.E.M.; Cells, S. MXene based new class of silicone oil nanofluids for the performance improvement of concentrated photovoltaic thermal collector. Sol. Energy Mater. Sol. Cells 2020, 211, 110526. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab.: Lemont, IL, USA, 1995. [Google Scholar]
- Saidur, R.; Leong, K.Y.; Mohammed, H.A. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Iranmanesh, S.; Ong, H.C.; Ang, B.C.; Sadeghinezhad, E.; Esmaeilzadeh, A.; Mehrali, M. Thermal performance enhancement of an evacuated tube solar collector using graphene nanoplatelets nanofluid. J. Clean. Prod. 2017, 162, 121–129. [Google Scholar] [CrossRef]
- Amani, M.; Amani, P.; Mahian, O.; Estellé, P. Multi-objective optimization of thermophysical properties of eco-friendly organic nanofluids. J. Clean. Prod. 2017, 166, 350–359. [Google Scholar] [CrossRef]
- Sarkar, J. A critical review on convective heat transfer correlations of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 3271–3277. [Google Scholar] [CrossRef]
- Wang, X.Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirlohi, A.; Nazari, M.A.; Ghasempour, R. A review of thermal conductivity of various nanofluids. J. Mol. Liq. 2018, 265, 181–188. [Google Scholar] [CrossRef]
- Parashar, N.; Aslfattahi, N.; Yahya, S.M.; Saidur, R. An Artificial Neural Network Approach for the Prediction of Dynamic Viscosity of MXene-Palm Oil Nanofluid Using Experimental Data; Springer: Berlin, Germany, 2020. [Google Scholar]
- Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 2014, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Mesgari, S.; Taylor, R.A.; Hjerrild, N.E.; Crisostomo, F.; Li, Q.; Scott, J. An investigation of thermal stability of carbon nanofluids for solar thermal applications. Sol. Energy Mater. Sol. Cells 2016, 157, 652–659. [Google Scholar] [CrossRef]
- Yashawantha, K.M.; Asif, A.; Babu, G.R.; Ramis, M.K. Rheological Behavior and Thermal Conductivity of Graphite–Ethylene Glycol Nanofluid. J. Test. Eval. 2019, 49, 4. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. Application of large-scale prepared MWCNTs nanofluids in solar energy system as volumetric solar absorber. Sol. Energy Mater. Sol. Cells 2019, 200, 109931. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Almuqati, T.; Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes. J. Clean. Prod. 2018, 191, 429–435. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Akhiani, A.R.; Latibari, S.T.; Talebian, S.; Dolatshahi-Pirouz, A.; Metselaar, H.S.; Mehrali, M. An ecofriendly graphene-based nanofluid for heat transfer applications. J. Clean. Prod. 2016, 137, 555–566. [Google Scholar] [CrossRef]
- Hajjar, Z.; morad Rashidi, A.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D.; Johnson, W.; Nawaz, K.; Cunningham, G. Thermo-physical properties of diamond nanofluids: A review. Int. J. Heat Mass Transf. 2019, 129, 1123–1135. [Google Scholar] [CrossRef]
- Zheng, R.; Gao, J.; Wang, J.; Feng, S.P.; Ohtani, H.; Wang, J.; Chen, G. Thermal Percolation in Stable Graphite Suspensions. Nano Lett. 2012, 12, 188–192. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Bao, D.J.N. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2009, 21, 055705. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Wang, F.; Han, L.; Zhang, Z.; Fang, X.; Shi, J.; Ma, W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res. Lett. 2012, 7, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.; Elsinawi, A. Thermal Conductivity Enhancement Phenomena in Ionic Liquid-Based Nanofluids (Ionanofluids). Aust. J. Chem. 2019, 72, 21–33. [Google Scholar] [CrossRef]
- Hosseinghorbani, A.; Mozaffarian, M.; Pazuki, G. Application of graphene oxide IoNanofluid as a superior heat transfer fluid in concentrated solar power plants. Int. Commun. Heat Mass Transf. 2020, 111, 104450. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Qeays, I.A.; Yahya, S.M.; Asjad, M.; Khan, Z.A. Multi-performance optimization of nanofluid cooled hybrid photovoltaic thermal system using fuzzy integrated methodology. J. Clean. Prod. 2020, 256, 120451. [Google Scholar] [CrossRef]
- Abdallah, S.R.; Saidani-Scott, H.; Abdellatif, O.E. Performance analysis for hybrid PV/T system using low concentration MWCNT (water-based) nanofluid. Sol. Energy 2019, 181, 108–115. [Google Scholar] [CrossRef]
- Aberoumand, S.; Ghamari, S.; Shabani, B. Energy and exergy analysis of a photovoltaic thermal (PV/T) system using nanofluids: An experimental study. Sol. Energy 2018, 165, 167–177. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M.; Heris, S.Z. Experimental investigation of the effects of silica/water nanofluid on PV/T (photovoltaic thermal units). Energy 2014, 66, 264–272. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M.; Maghrebi, M.-J.; Ghazikhani, M. Experimental study of using both ZnO/water nanofluid and phase change material (PCM) in photovoltaic thermal systems. Sol. Energy Mater. Sol. Cells 2017, 161, 62–69. [Google Scholar] [CrossRef]
- Ebaid, M.S.Y.; Ghrair, A.M.; Al-Busoul, M. Experimental investigation of cooling photovoltaic (PV) panels using (TiO2) nanofluid in water -polyethylene glycol mixture and (Al2O3) nanofluid in water- cetyltrimethylammonium bromide mixture. Energy Convers. Manag. 2018, 155, 324–343. [Google Scholar] [CrossRef]
- Hendricks, J.H.; Van Sark, W.G. Annual performance enhancement of building integrated photovoltaic modules by applying phase change materials. Progress in Photovoltaics: Research and Applications. Prog. Photovolt. Res. Appl. 2013, 21, 620–630. [Google Scholar]
- Ghadiri, M.; Sardarabadi, M.; Pasandideh-Fard, M.; Moghadam, A.J. Experimental investigation of a PVT system performance using nano ferrofluids. Energy Convers. Manag. 2015, 103, 468–476. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lin, Y.; Hu, D.; Jiang, P.; Huang, X. Multifunctional 3D-MXene/PDMS nanocomposites for electrical, thermal and triboelectric applications. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105754. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 5109–5124. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Lei, J.C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Ji, J.; Zhao, L.; Shen, Y.; Liu, S.; Zhang, Y. Covalent stabilization and functionalization of MXene via silylation reactions with improved surface properties. FlatChem 2019, 17, 100128. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Wang, X.; Garnero, C.; Rochard, G.; Magne, D.; Morisset, S.; Hurand, S.; Chartier, P.; Rousseau, J.; Cabioc’h, T.; Coutanceau, C.; et al. A new etching environment (FeF3/HCl) for the synthesis of two-dimensional titanium carbide MXenes: A route towards selective reactivity vs. water. J. Mater. Chem. A 2017, 5, 22012–22023. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, X.; Gong, S.; Wei, S.; Liu, J.; Xu, Y. Solvent-regulated preparation of well-intercalated Ti3C2Tx MXene nanosheets and application for highly effective electromagnetic wave absorption. Nanotechnology 2018, 29, 355201. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Zhang, M.; Li, M.; Gao, Y.; Wang, C.; Chen, G.; Du, F. Ti3C2T x MXene decorated with Sb nanoparticles as anodes material for sodium-ion batteries. Nanotechnology 2019, 30, 134001. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Wang, J.; Deng, Q.; Li, M.; Du, S.; Han, Y.H.; Lee, J.; Huang, Q. Facile preparation of in situ coated Ti3C2Tx/Ni0.5Zn0.5Fe2O4 composites and their electromagnetic performance. RSC Adv. 2017, 7, 24698–24708. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, A.; Thynell, S.T. Confined rapid thermolysis/FTIR/ToF studies of imidazolium-based ionic liquids. Thermochim. Acta 2006, 443, 159–172. [Google Scholar] [CrossRef]
- Arifutzzaman, A.; Ismail, A.F.; Yaacob, I.I.; Alam, M.Z.; Khan, A.A. Stability investigation of water based exfoliated graphene nanofluids. IOP Conf. Ser. Mater. Sci. Eng. 2019, 488, 012002. [Google Scholar] [CrossRef] [Green Version]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef] [Green Version]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin, Germany, 2011; pp. 63–70. [Google Scholar]
- Han, R.; Ma, X.; Xie, Y.; Teng, D.; Zhang, S. Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux. RSC Adv. 2017, 7, 56204–56210. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, S.; Kim, M.; Blanch, H.W.; Prausnitz, J.M. Solubility and rate of dissolution for Miscanthus in hydrophilic ionic liquids. Fluid Phase Equilibria 2011, 309, 89–96. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.; Mirshekari, G.; Davidson, J.; Kerns, D. Nanodiamond nanofluid microstructural and thermo-electrical characterization. Int. Commun. Heat Mass Transf. 2019, 101, 82–88. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, M. Characterisation and Zeta Potential Measurements of CuO–Water Nanofluids. In Advances in Interdisciplinary Engineering; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2019; pp. 741–747. [Google Scholar]
- Said, Z.; Abdelkareem, M.A.; Rezk, H.; Nassef, A.M.; Atwany, H.Z. Stability, thermophysical and electrical properties of synthesized carbon nanofiber and reduced-graphene oxide-based nanofluids and their hybrid along with fuzzy modeling approach. Powder Technol. 2020, 364, 795–809. [Google Scholar] [CrossRef]
- Chen, W.; Qiu, L.; Liang, S.; Zheng, X.; Tang, D. Measurement of thermal conductivities of [mmim]DMP/CH3OH and [mmim]DMP/H2O by freestanding sensor-based 3ω technique. Thermochimica Acta 2013, 560, 1–6. [Google Scholar] [CrossRef]
- Akhavan-Zanjani, H.; Saffar-Avval, M.; Mansourkiaei, M.; Ahadi, M.; Sharif, F. Turbulent Convective Heat Transfer and Pressure Drop of Graphene–Water Nanofluid Flowing Inside a Horizontal Circular Tube. J. Dispers. Sci. Technol. 2014, 35, 1230–1240. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Lee, G.-J.; Rhee, C.K. Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. J. Mater. Sci. 2014, 4, 1506–1511. [Google Scholar] [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. Enhanced specific heat and thermal conductivity of ternary carbonate nanofluids with carbon nanotubes for solar power applications. Int. J. Energy Res. 2020, 44, 334–343. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Ghazvini, M.; Akhavan-Behabadi, M.A.; Rasouli, E.; Raisee, M. Heat transfer properties of nanodiamond–engine oil nanofluid in laminar flow. Heat Transf. Eng. 2012, 33, 525–532. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; Elsinawi, A. Understanding the heat capacity enhancement in ionic liquid-based nanofluids (ionanofluids). J. Mol. Liq. 2018, 253, 326–339. [Google Scholar] [CrossRef]
- Elhamarnah, Y.A.; Nasser, M.; Qiblawey, H.; Benamor, A.; Atilhan, M.; Aparicio, S. A comprehensive review on the rheological behavior of imidazolium based ionic liquids and natural deep eutectic solvents. J. Mol. Liq. 2019, 277, 932–958. [Google Scholar] [CrossRef]
- Aravind, S.S.J.; Baskar, P.; Baby, T.T.; Sabareesh, R.K.; Das, S.; Ramaprabhu, S. Investigation of Structural Stability, Dispersion, Viscosity, and Conductive Heat Transfer Properties of Functionalized Carbon Nanotube Based Nanofluids. J. Phys. Chem. C 2011, 115, 16737–16744. [Google Scholar] [CrossRef]
- Ko, G.H.; Heo, K.; Lee, K.; Kim, D.S.; Kim, C.; Sohn, Y.; Choi, M. An experimental study on the pressure drop of nanofluids containing carbon nanotubes in a horizontal tube. Int. J. Heat Mass Transf. 2007, 50, 4753. [Google Scholar] [CrossRef]
- Adam, S.A.; Ju, X.; Zhang, Z.; Lin, J.; El-Samie, M.M.A.; Xu, C. Effect of temperature on the stability and optical properties of SiO2-water nanofluids for hybrid photovoltaic/thermal applications. Appl. Therm. Eng. 2020, 175, 115394. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M. Experimental and numerical study of metal-oxides/water nanofluids as coolant in photovoltaic thermal systems (PVT). Sol. Energy Mater. Sol. Cells 2016, 157, 533–542. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, S.G.; Lee, G.H. Efficiency Improvement of a Photovoltaic Thermal (PVT) System Using Nanofluids. Energies 2019, 12, 3063. [Google Scholar] [CrossRef] [Green Version]














| Property | [MMIM][DMP] |
|---|---|
| Purity (HPLC) | ≥98.0% |
| Identity (NMR) | passed |
| Density | 1.27 g/cm3 (20 °C) |
| Water (KF) | ≤0.1% |
| Halides (IC) | ≤0.1% |
| Color | Yellow |
| Standard Sample | Kmeasured | Kreference Wm−1k−1 | Standard Deviation σk |
|---|---|---|---|
| Glycerin (20 °C) | 0.289 | 0.282 | ±0.038 |
| Make and Model No. | Vikram Solar, ELDORA VSP.72.AAA.03 |
|---|---|
| Material | Polycrystalline Silicon Cell |
| Dimension | 1955 × 982 × 36 mm |
| No. of cells | 72 |
| Peak power | 300 W |
| Maximum Voltage (Vmpp) | 37.05 V |
| Maximum Current (Impp) | 8.10 A |
| Open circuit voltage (Voc) | 45.58 V |
| Short circuit current (Isc) | 8.58 A |
| Weight of PV module | 20.5 Kg |
| Operating temperature range | −40 °C to +85 °C |
| Standard test condition (STC) | 1000 W/m2, AM 1.5, 25 °C |
| Properties | Values |
| Heat transfer coefficient from Panel to tedlar | 150W/m2K |
| Heat transfer coefficient from tedlar to tubing | 77 W/m2K |
| Heat transfer coefficient from tubing to nanofluid | 66 W/m2K |
| Absorptivity of PV module | 0.9 |
| Absorptivity of tedlar sheet | 0.5 |
| Emissivity of PV panel | 0.99 |
| Thermal conductivity of EVA | 0.311 W/m-K |
| Thermal conductivity solar panel | 148 W/m-K |
| Thermal conductivity of tedlar | 0.15 W/m-K |
| Thermal conductivity of thermal paste | 1.9 W/m-K |
| Thermal conductivity of tubes | 2700 W/m-K |
| S.No. | Mesh Size (no. of Elements) | Panel Temperature (℃) | % Deviation | Outlet Temperature (℃) | % Deviation | Time of Solution (s) |
|---|---|---|---|---|---|---|
| 1 | 2.5 × 105 | 42.341 | - | 41.213 | - | 560 |
| 2 | 4 × 105 | 43.872 | 1.2% | 40.751 | −1.13% | 720 |
| 3 | 6 × 105 | 44.003 | 0.29% | 40.254 | −1.23% | 817 |
| 4 | 8 × 105 | 44.118 | 0.26% | 39.104 | −2.94% | 1115 |
| 5 | 1.5 × 106 | 45.200 | 2.3% | 38.889 | −0.55% | 1487 |
| 6 | 3.5 × 106 | 45.201 | 0.002% | 38.801 | −0.22% | 1815 |
| Concentration | Zeta Potential (mV) | |||||
|---|---|---|---|---|---|---|
| 25 °C | Uncertainty (%) | 45 °C | Uncertainty (%) | 60 °C | Uncertainty (%) | |
| 0.05 | −18.33 | <5 | −29.52 | <5 | −38.68 | <5 |
| 0.10 | −19.16 | <5 | −34.64 | <5 | −39.54 | <5 |
| 0.20 | −17.88 | <5 | −32.15 | <5 | −35.35 | <5 |
| Panel temperature (°C) | Percentage Error | Remark | |
|---|---|---|---|
| Present Research | Sardarabadi et al. [83] | ||
| 56.35 | 56.21 | 0.25% | At 1000 W/m2 and at a flow rate of 0.025kg/s (Numerical study of [83]) |
| Electrical efficiency | |||
| Present research | Lee et al. [84] | ||
| 12.15 | 12.22 | 0.5% | At 1000 W/m2 and at a flow rate of 0.05kg/s (Experimental study of [84]) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials 2020, 10, 1372. https://doi.org/10.3390/nano10071372
Das L, Habib K, Saidur R, Aslfattahi N, Yahya SM, Rubbi F. Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials. 2020; 10(7):1372. https://doi.org/10.3390/nano10071372
Chicago/Turabian StyleDas, Likhan, Khairul Habib, R. Saidur, Navid Aslfattahi, Syed Mohd Yahya, and Fazlay Rubbi. 2020. "Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System" Nanomaterials 10, no. 7: 1372. https://doi.org/10.3390/nano10071372
APA StyleDas, L., Habib, K., Saidur, R., Aslfattahi, N., Yahya, S. M., & Rubbi, F. (2020). Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials, 10(7), 1372. https://doi.org/10.3390/nano10071372