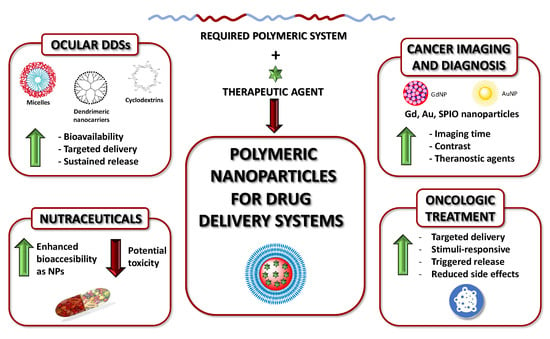
Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects
Abstract
:1. Introduction
2. Polymeric Nanocarriers for Ocular Drug Delivery
2.1. Micelle Nanocarriers for Ocular Delivery
2.2. Dendrimeric Nanocarriers for Ocular Delivery
2.3. Other Types of Polymeric Nanocarriers for Ocular Delivery
3. Polymeric Nanoparticles in Cancer Diagnosis and Imaging
3.1. Gold-Based Polymeric Nanoparticles Used in Cancer Diagnosis
3.2. Gadolinium Polymeric Nanoparticles (GdNPs) Used in Cancer Diagnosis
3.3. Perfluorocarbons Polymeric Nanoparticles (PFCNPs) Used in Cancer Diagnosis
3.4. Other Nanoparticles Used in Cancer Diagnosis
4. Polymeric Nanoparticles in Oncologic Treatment
4.1. Advantages of Nanotechnological Drug-Delivery Systems
4.2. Challenges Associated with Nanoparticulate Drug-Delivery Systems
4.3. The Enhanced Permeability and Retention (EPR) Effect
4.4. Active Targeting
4.5. Stimuli-Responsive and Triggered Release Systems
5. Polymeric Nanoparticles as Nutraceutical Agents
5.1. Bioavailability and Nanoparticles
5.2. Toxicity
6. Future Challenges in DDS
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; Capter 6; pp. 87–194. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Role of Nanobiotechnology in Drug Delivery. Methods Mol. Biol. 2020, 2059, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Zhang, J.S.; Yang, F.; Zhou, Q.L.; Liao, Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly (lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-Step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, e1802851. [Google Scholar] [CrossRef]
- Pla, D.; Gómez, M. Metal and Metal Oxide Nanoparticles: A Lever for C–H Functionalization. ACS Catal. 2016, 6, 3537–3552. [Google Scholar] [CrossRef]
- Begines, B.; de-Paz, M.-V.; Alcudia, A.; Galbis, J.A. Synthesis of reduction sensitive comb-like polyurethanes using click chemistry. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3888–3900. [Google Scholar] [CrossRef]
- He, Y.F.; Zhang, F.; Saleh, E.; Vaithilingam, J.; Aboulkhair, N.; Begines, B.; Tuck, C.J.; Hague, R.J.M.; Ashcroft, I.A.; Wildman, R.D. A Tripropylene Glycol Diacrylate-based Polymeric Support Ink for Material Jetting. Addit. Manuf. 2017, 16, 153–161. [Google Scholar] [CrossRef]
- Begines, B.; Zamora, F.; Violante de Paz, M.; Roffe, I.; Mancera, M.; Galbis, J.A. Synthesis and Characterization of New Carbohydrate-based Polyureas. J. Renew. Mater. 2013, 1, 212–221. [Google Scholar] [CrossRef]
- Begines, B.; Alcudia, A.; Aguilera-Velazquez, R.; Martinez, G.; He, Y.; Wildman, R.; Sayagues, M.J.; Jimenez-Ruiz, A.; Prado-Gotor, R. Design of highly stabilized nanocomposite inks based on biodegradable polymer-matrix and gold nanoparticles for Inkjet Printing. Sci. Rep. 2019, 9, 16097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology. Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–244. [Google Scholar]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775–791. [Google Scholar] [CrossRef]
- Belletti, D.; Grabrucker, A.M.; Pederzoli, F.; Menrath, I.; Cappello, V.; Vandelli, M.A.; Forni, F.; Tosi, G.; Ruozi, B. Exploiting The Versatility of Cholesterol in Nanoparticles Formulation. Int. J. Pharm. 2016, 511, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Musumeci, T.; Carbone, C.; Vicari, L.; Lauro, M.R.; Puglisi, G. Revisiting the role of sucrose in PLGA-PEG nanocarrier for potential intranasal delivery. Pharm. Dev. Technol. 2018, 23, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Saha, R.N. Investigations on pharmacokinetics and biodistribution of polymeric and solid lipid nanoparticulate systems of atypical antipsychotic drug: Effect of material used and surface modification. Drug Dev. Ind. Pharm. 2017, 43, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Thompson, D.H.; Park, K.; Li, T. Impact of surfactant treatment of paclitaxel nanocrystals on biodistribution and tumor accumulation in tumor-bearing mice. J. Control. Release 2016, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Yin, J.; Deng, X.; Zhang, J.; Lin, J. Current Understanding of Interactions between Nanoparticles and ABC Transporters in Cancer Cells. Curr. Med. Chem. 2018, 25, 5930–5944. [Google Scholar] [CrossRef]
- World Health Organization. Blindness and Vision Impairment. Available online: https://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 20 March 2020).
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Neumann, R.; Barequet, D. The gap between the need for novel retinal drug delivery methods, technologies in R&D phase, and approved ocular drug delivery technologies. Drug Discov. Today 2019, 24, 1433–1435. [Google Scholar] [CrossRef]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Juliana, F.R.; Kesse, S.; Boakye-Yiadom, K.O.; Veroniaina, H.; Wang, H.; Sun, M. Promising Approach in the Treatment of Glaucoma Using Nanotechnology and Nanomedicine-Based Systems. Molecules 2019, 24, 3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazaki, S.; Pachis, K.; Tzatzarakis, M.; Tsilimbaris, M.; Antimisiaris, S.G. Novel Liposome Aggregate Platform (LAP) system for sustained retention of drugs in the posterior ocular segment following intravitreal injection. Int. J. Pharm. 2020, 576, 118987. [Google Scholar] [CrossRef]
- Dukovski, B.J.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Crespo Moral, M.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979. [Google Scholar] [CrossRef]
- Kim, H.-J.; Zhang, K.; Moore, L.; Ho, D. Diamond Nanogel-Embedded Contact Lenses Mediate Lysozyme-Dependent Therapeutic Release. ACS Nano 2014, 8, 2998–3005. [Google Scholar] [CrossRef]
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of cationic nanocrystals for ocular delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222. [Google Scholar] [CrossRef]
- Chi, H.; Gu, Y.; Xu, T.; Cao, F. Multifunctional organic–inorganic hybrid nanoparticles and nanosheets based on chitosan derivative and layered double hydroxide: Cellular uptake mechanism and application for topical ocular drug delivery. Int. J. Nanomed. 2017, 12, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.-R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. A 2015, 103A, 3148–3156. [Google Scholar] [CrossRef]
- Ryu, W.M.; Kim, S.-N.; Min, C.H.; Choy, Y.B. Dry Tablet Formulation of PLGA Nanoparticles with a Preocular Applicator for Topical Drug Delivery to the Eye. Pharmaceutics 2019, 11, 651. [Google Scholar] [CrossRef] [Green Version]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-RelatedMacular Degeneration. Molecualr Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Li, G.; Xu, F.; Li, S.; Teng, L.; Li, Y.; Sun, F. Anti-Angiogenic Activity Of Bevacizumab-Bearing Dexamethasone-Loaded PLGA Nanoparticles ForPotential Intravitreal Applications. Int. J. Nanomed. 2019, 14, 8819–8834. [Google Scholar] [CrossRef] [Green Version]
- Salama, H.A.; Ghorab, M.; Mahmoud, A.A.; Abdel Hady, M. PLGA Nanoparticles as Subconjunctival Injection for Management of Glaucoma. AAPS PharmSciTech 2017, 18, 2517–2528. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Q.; Boylan, N.J.; Lamb, N.W.; Emmert, D.G.; Yang, J.-C.; Tang, L.; Heflin, T.; Alwadani, S.; Eberhart, C.G.; et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J. Control. Release 2015, 201, 32–40. [Google Scholar] [CrossRef]
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled Release of Bevacizumab through Nanospheres for Extended Treatment of Age-Related Macular Degeneration. Open Ophthalmol. J. 2012, 6, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Imam, S.S.; Bukhari SN, A.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosannanoparticle for ocular delivery: In-vitro characterization, oculartolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. A 2018, 106, 2261–2271. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [Green Version]
- Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G.; Alqurshi, A. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679–3689. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drugdelivery of dexamethasone. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan grafted methoxy poly(ethylene glycol)-poly(e-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci. Rep. 2015, 5, 11337. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chen, D.; Li, Y.; Yang, W.; Tu, J.; Shen, Y. Improving the topical ocular pharmacokinetics of lyophilized cyclosporine A-loaded micelles: Formulation, in vitro and in vivo studies. Drug Deliv. 2019, 25, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Yin, L.; Zhang, Y.; Yu, W.; Wang, Q.; Zhan, Z. Preparation and study of two kinds of ophthalmic nano-preparations of everolimus. Drug Deliv. 2019, 26, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yu, X.; Zhou, Y.; Zhang, R.; Song, Q.; Wang, Q.; Li, X. Directing the nanoparticle formation by the combination with small molecular assembly and polymeric assembly for topical suppression of ocular inflammation. Int. J. Pharm. 2018, 551, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ban, J.; Mo, Z.; Zhang, Y.; An, P.; Liu, L.; Xie, Q.; Du, Y.; Xie, B.; Zhan, X.; et al. A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone. Nanotechnology 2018, 29, 425101. [Google Scholar] [CrossRef]
- Hirani, A.; Grover, A.; Lee, Y.W.; Pathak, Y.; Sutariya, V. Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for agerelated macular degeneration. Pharm. Dev. Technol. 2016, 21, 61–67. [Google Scholar] [CrossRef]
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in Porous Microparticles Prepared by Supercritical Infusion and Pressure Quench Technology for Sustained Delivery of Bevacizumab. Mol. Pharm. 2012, 10, 4676–4686. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wanga, A.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Lancina, M.G.; Wang, J.; Williamson, G.S.; Yang, H. DenTimol as A Dendrimeric Timolol Analogue for Glaucoma Therapy: Synthesis and Preliminary Efficacy and Safety Assessment. Mol. Pharm. 2018, 15, 2883–2889. [Google Scholar] [CrossRef]
- Tai, L.; Liu, C.; Jiang, K.; Chen, X.; Feng, L.; Pan, W.; Wei, G.; Lu, W. A novel penetratin-modified complex for noninvasive intraocular delivery of antisense oligonucleotides. Int. J. Pharm. 2017, 529, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.P.; Kannan, R.M. Dendrimer Nanoparticles for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Yu, G.; Luo, C.; Maeda, A.; Zhang, N.; Sun, D.; Zhou, Z.; Puntel, A.; Palczewski, K.; Lu, Z.-R. Synthesis and evaluation of a nanoglobular dendrimer 5- aminosalicylic acid conjugate with a hydrolyzable chiff base spacer for treating retinal degeneration. ACS Nano 2014, 8, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Spataro, G.; Malecaze, F.; Turrin, C.-O.; Soler, V.; Duhayon, C.; Elena, P.-P.; Majoral, J.-P.; Caminade, A.-M. Designing dendrimers for ocular drug delivery. Eur. J. Med. Chem. 2010, 45, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 1909049. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guinchard, S.; Guillarme, D.; Pupier, M.; Jeannerat, D.; Rivara-Minten, E.; Veuthey, J.L.; Gurny, R. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur. J. Pharm. Biopharm. 2015, 95, 203–214. [Google Scholar] [CrossRef]
- Jansook, P.; Kulsirachote, P.; Loftsson, T. Cyclodextrin solubilization of celecoxib: Solid and solution state characterization. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 75–88. [Google Scholar] [CrossRef]
- Jansook, P.; Kulsirachote, P.; Asasutjarit, R.; Loftsson, T. Development of celecoxib eye drop solution and microsuspension: A comparative investigation of binary and ternary cyclodextrin complexes. Carbohydr. Polym. 2019, 225, 115209. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin-Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Farace, C.; Sánchez-Moreno, P.; Orecchioni, M.; Manetti, R.; Sgarrella, F.; Asara, Y.; Peula-García, J.M.; Marchal, J.A.; Madeddu, R.; Delogu, L.G. Immune cell impact of three differently coated lipid nanocapsules: Pluronic, chitosan and polyethylene glycol. Sci. Rep. 2016, 6, 18423. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Schuh, R.S.; Maschio, V.J.; Brazil, N.T.; Rott, M.B.; Teixeira, H.F. Box Behnken design of siRNA-loaded liposomes for the treatment of amurine model of ocular keratitis caused byAcanthamoeba. Colloids Surf. B Biointerfaces 2019, 173, 725–732. [Google Scholar] [CrossRef]
- Patil, A.; Lakhani, P.; Taskar, P.; Wu, K.-W.; Sweeney, C.; Avula, B.; Wang, Y.-H.; Khan, I.A.; Majumdar, S. Formulation Development, Optimization, and In vitro—In vivo Characterization of Natamycin Loaded PEGylated Nano-lipid Carriers for Ocular Applications. J. Pharm. Sci. 2018, 107, 2160–2171. [Google Scholar] [CrossRef]
- Fathi Karkan, S.; Mohammadhosseini, M.; Panahi, Y.; Milani, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, E.; Hosseini, A.; Davaran, S. Magnetic nanoparticles in cancer diagnosis and treatment: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–5. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Tay, Z.W.; Chandrasekharan, P.; Yu, E.Y.; Hensley, D.W.; Orendorff, R.; Jeffris, K.E.; Mai, D.; Zheng, B.; Goodwill, P.W.; et al. Magnetic particle imaging for radiation-free, sensitive and high-contrast vascular imaging and cell tracking. Curr. Opin. Chem. Biol. 2018, 45, 131–138. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Optical, electrochemical and catalytic methods for in-vitro diagnosis using carbonaceous nanoparticles: A review. Microchim. Acta 2019, 186, 50. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Maham, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276. [Google Scholar]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling Polymer-Coated Gold Nanoparticles as a Contrast Agent for in Vivo X-ray Computed Tomography Imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef]
- Rabin, O.; Manuel Perez, J.; Grimm, J.; Wojtkiewicz, G.; Weissleder, R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater. 2006, 5, 118–122. [Google Scholar] [CrossRef]
- Al Zaki, A.; Joh, D.; Cheng, Z.; De Barros, A.L.B.; Kao, G.; Dorsey, J.; Tsourkas, A. Gold-Loaded Polymeric Micelles for Computed Tomography-Guided Radiation Therapy Treatment and Radiosensitization. ACS Nano 2014, 8, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Zhang, X.F.; Qian, L.; Yao, N.; Pan, Y.; Zhang, L.J. Doxorubicin-Loaded Unimolecular Micelle-Stabilized Gold Nanoparticles as a Theranostic Nanoplatform for Tumor-Targeted Chemotherapy and Computed Tomography Imaging. Biomacromolecules 2017, 18, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, J.; Kim, S.; Kim, S.-H.; Lee, T.G.; Lee, J.Y.; Wi, J.-S. Characterization and application of porous gold nanoparticles as 2-photon luminescence imaging agents: 20-fold brighter than gold nanorods. J. Biophotonics 2018, 11, e201700174. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.D.; Pearson, J.R.; Leal, M.P.; Torres, M.J.; Blanca, M.; Mayorga, C.; Le Guével, X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials 2015, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; He, Y.; Liu, X.-L.; Lin, M.-L.; Cheng, Y.; Zhao, J.; Gong, Q.; Xia, K.; Tan, P.-H.; Lu, G. Spectral shape of one-photon luminescence from single gold nanorods. AIP Adv. 2017, 7, 125106. [Google Scholar] [CrossRef]
- Wang, Y.; Strohm, E.M.; Sun, Y.; Wang, Z.; Zheng, Y.; Wang, Z.; Kolios, M.C. Biodegradable polymeric nanoparticles containing gold nanoparticles and Paclitaxel for cancer imaging and drug delivery using photoacoustic methods. Biomed. Opt. Express 2016, 7, 4125–4138. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Weissleder, R. Molecular Imaging in Cancer. Science 2006, 312, 1168. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010, 5, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Ratzinger, G.; Agrawal, P.; Körner, W.; Lonkai, J.; Sanders, H.M.H.F.; Terreno, E.; Wirth, M.; Strijkers, G.J.; Nicolay, K.; Gabor, F. Surface modification of PLGA nanospheres with Gd-DTPA and Gd-DOTA for high-relaxivity MRI contrast agents. Biomaterials 2010, 31, 8716–8723. [Google Scholar] [CrossRef]
- Caravan, P.; Zhang, Z. Targeted MRI Contrast Agents. Chem. Contrast Agents Med Magn. Reson. Imaging 2013. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, C.; Yu, D.; Lu, Z.; Zhang, N. Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials 2011, 32, 5167–5176. [Google Scholar] [CrossRef]
- Esser, L.; Truong, N.P.; Karagoz, B.; Moffat, B.A.; Boyer, C.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Gadolinium-functionalized nanoparticles for application as magnetic resonance imaging contrast agents via polymerization-induced self-assembly. Polym. Chem. 2016, 7, 7325–7337. [Google Scholar] [CrossRef]
- Kim, C.; Song, K.H.; Gao, F.; Wang, L.V. Sentinel lymph nodes and lymphatic vessels: Noninvasive dual-modality in vivo mapping by using indocyanine green in rats—Volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology 2010, 255, 442–450. [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Beija, M.; Laurent, S.; Vander Elst, L.; Muller, R.; Duong, H.; Lowe, A.; Davis, T.; Boyer, C. Macromolecular Ligands for Gadolinium MRI Contrast Agents. Macromolecules 2012, 45, 4196–4204. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, R.; Wen, X.; Li, L.; Li, C. Micelles based on biodegradable poly (L-glutamic acid)-b-polylactide with paramagnetic Gd ions chelated to the shell layer as a potential nanoscale MRI-visible delivery system. Biomacromolecules 2008, 9, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, T.; Zhang, G.; Jin, F.; Liu, S. Synergistically Enhance Magnetic Resonance/Fluorescence Imaging Performance of Responsive Polymeric Nanoparticles under Mildly Acidic Biological Milieu. Macromol. Rapid Commun. 2013, 34, 749–758. [Google Scholar] [CrossRef]
- Li, A.; Luehmann, H.P.; Sun, G.; Samarajeewa, S.; Zou, J.; Zhang, S.; Zhang, F.; Welch, M.J.; Liu, Y.; Wooley, K.L. Synthesis and In Vivo Pharmacokinetic Evaluation of Degradable Shell Cross-Linked Polymer Nanoparticles with Poly(carboxybetaine) versus Poly(ethylene glycol) Surface-Grafted Coatings. ACS Nano 2012, 6, 8970–8982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi-Gahrouei, D.; Williams, M.; Rizvi, S.; Allen, B.J. In vivo studies of Gd-DTPA-monoclonal antibody and gd-porphyrins: Potential magnetic resonance imaging contrast agents for melanoma. J. Magn. Reson. Imaging 2001, 14, 169–174. [Google Scholar] [CrossRef]
- Hu, X.; Lu, F.; Chen, L.; Tang, Y.; Hu, W.; Lu, X.; Ji, Y.; Yang, Z.; Zhang, W.; Yin, C.; et al. Perylene Diimide-Grafted Polymeric Nanoparticles Chelated with Gd3+ for Photoacoustic/T1-Weighted Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30458–30469. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lu, S.-T.; Yu, H.; Liao, R.-F.; Li, H.; Lucie Zafitatsimo, B.V.; Li, Y.-S.; Zhang, Y.; Zhu, X.-L.; Liu, H.-G.; et al. Gadolinium-chelate functionalized bismuth nanotheranostic agent for in vivo MRI/CT/PAI imaging-guided photothermal cancer therapy. Biomaterials 2018, 159, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W.M. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef]
- Cyrus, T.; Winter, P.M.; Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Magnetic resonance nanoparticles for cardiovascular molecular imaging and therapy. Expert Rev. Cardiovasc. Ther. 2005, 3, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Edwards, P.G.; Paisey, S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011, 1, 1415–1425. [Google Scholar] [CrossRef]
- Wek, K.S. Development of Polymeric Therapeutic Nanoparticles: Toward Targeted Delivery and Efficient 19F MRI of Solid Tumors. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2017. [Google Scholar]
- Wallat, J.D.; Czapar, A.E.; Wang, C.; Wen, A.M.; Wek, K.S.; Yu, X.; Steinmetz, N.F.; Pokorski, J.K. Optical and Magnetic Resonance Imaging Using Fluorous Colloidal Nanoparticles. Biomacromolecules 2017, 18, 103–112. [Google Scholar] [CrossRef]
- Pisani, E.; Tsapis, N.; Paris, J.; Nicolas, V.; Cattel, L.; Fattal, E. Polymeric Nano/Microcapsules of Liquid Perfluorocarbons for Ultrasonic Imaging: Physical Characterization. Langmuir 2006, 22, 4397–4402. [Google Scholar] [CrossRef]
- Giraudeau, C.; Flament, J.; Marty, B.; Boumezbeur, F.; Mériaux, S.; Robic, C.; Port, M.; Tsapis, N.; Fattal, E.; Giacomini, E.; et al. A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn. Reson. Med. 2010, 63, 1119–1124. [Google Scholar] [CrossRef]
- Diou, O.; Fattal, E.; Delplace, V.; Mackiewicz, N.; Nicolas, J.; Mériaux, S.; Valette, J.; Robic, C.; Tsapis, N. RGD decoration of PEGylated polyester nanocapsules of perfluorooctyl bromide for tumor imaging: Influence of pre or post-functionalization on capsule morphology. Eur. J. Pharm. Biopharm. 2014, 87, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liopo, A.; Su, R.; Oraevsky, A.A. Melanin nanoparticles as a novel contrast agent for optoacoustic tomography. Photoacoustics 2015, 3, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belletti, D.; Riva, G.; Luppi, M.; Tosi, G.; Forni, F.; Vandelli, M.; Pederzoli, F. Anticancer drug-loaded quantum dots engineered polymeric nanoparticles: Diagnosis/therapy combined approach. Eur. J. Pharm. Sci. 2017, 107. [Google Scholar] [CrossRef]
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Mignani, S.; Majoral, J.-P.; Shi, X. Targeted tumor dual mode CT/MR imaging using multifunctional polyethylenimine-entrapped gold nanoparticles loaded with gadolinium. Drug Deliv. 2018, 25, 178–186. [Google Scholar] [CrossRef]
- McQuade, C.; Al Zaki, A.; Desai, Y.; Vido, M.; Sakhuja, T.; Cheng, Z.; Hickey, R.J.; Joh, D.; Park, S.-J.; Kao, G.; et al. A Multifunctional Nanoplatform for Imaging, Radiotherapy, and the Prediction of Therapeutic Response. Small 2015, 11, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Carregal-Romero, S.; Barbosa, S.; Parak, W.J.; Taboada, P.; Mosquera, V. Polymeric-Gold Nanohybrids for Combined Imaging and Cancer Therapy. Adv. Healthc. Mater. 2014, 3, 1309–1325. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. Part A 2007, 80A, 333–341. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Jin, W.; Wu, F.; Zhang, Q.; Zhang, M.; Zhou, N.; Shen, J. Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@carbon nanoparticles. Anal. Chim. Acta 2019, 1056, 108–116. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, J.; Yan, Y.; Li, J.; Shen, M.; Zhang, G.; Mignani, S.; Shi, X. RGD-functionalized ultrasmall iron oxide nanoparticles for targeted T1-weighted MR imaging of gliomas. Nanoscale 2015, 7, 14538–14546. [Google Scholar] [CrossRef] [PubMed]
- Pernia Leal, M.; Rivera-Fernández, S.; Franco, J.M.; Pozo, D.; de la Fuente, J.M.; García-Martín, M.L. Long-circulating PEGylated manganese ferrite nanoparticles for MRI-based molecular imaging. Nanoscale 2015, 7, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Trans. Med. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomedicine 2016, 12, 81–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyamvada, P.; Chandana, M.; Sahoo, S. Nanotechnology-based combinational drug delivery:an emerging approach for cancer therapy. Drug Discos. Today 2012, 17. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.U.; Nie, S.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wong, S.; Chen, F.; Chang, T.; Lu, H.; Stenzel, M.H. Influencing Selectivity to Cancer Cells with Mixed Nanoparticles Prepared from Albumin–Polymer Conjugates and Block Copolymers. Bioconjugate Chem. 2017, 28, 979–985. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharm. Res. 2020, 43, 118–133. [Google Scholar] [CrossRef] [PubMed]
- John, T.A.; Vogel, S.M.; Minshall, R.D.; Ridge, K.; Tiruppathi, C.; Malik, A.B. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J. Physiol. 2001, 533, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Preeti, K.; Balaram, G.; Swati, B. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2015. [Google Scholar] [CrossRef]
- Fang, J.; Islam, R.; Islam, W.; Yin, H.; Subr, V.; Etrych, T.; Ulbrich, K.; Maeda, H. Augmentation of EPR Effect and Efficacy of Anticancer Nanomedicine by Carbon Monoxide Generating Agents. Pharmaceutics 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H. Toward a full understanding towards EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015. [Google Scholar] [CrossRef]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjug. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J. Enhancement of oral bioavailability of doxorubicin through surface modified biodegradable polymeric nanoparticles. Chem. Cent. J. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Soma, C.; Dubernet, C.; Bentolila, D.; Benita, S.; Couvreur, P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials 2002, 21, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, C.; Liu, M.; Pei, W.; Chu, X.; Zhang, H.; Wang, Z.; Han, J. An organic solvent-free technology for the fabrication of albumin-based paclitaxel nanoparticles for effective cancer therapy. Colloids Surf. B Biointerfaces 2019, 183, 110394. [Google Scholar] [CrossRef] [PubMed]
- Çirpanli, Y.; Allard, E.; Passirani, C.; Bilensoy, E.; Lemaire, L.; Çali, S.; Benoit, J. Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model. Int. J. Pharm. 2011, 403, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Jiang, X.; Yao, L.; Chen, J.; Chen, H. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkola, M.; Balabanyan, V.; et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–80. [Google Scholar] [CrossRef]
- Hekmatara, T.; Bernreuther, C.; Khalansky, A.; Theisen, A.; Weissenberger, J.; Matschke, J.; Gelperina, S.; Kreuter, J.; Glatzel, M. Efficient systemic therapy of rat glioblastoma by nanoparticle-bound doxorubicin is due to antiangiogenic effects. Clin. Neuropathol. 2009, 28, 153–164. [Google Scholar] [CrossRef]
- Khanna, V.; Kalscheuer, S.; Kirtane, A.; Zhang, W.; Panyam, J. Perlecan-targeted nanoparticles for drug delivery to triple-negative breast cancer. Future Drug Discov. 2019, 1, FDD8. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; ZhuGe, D.; Tong, M.; Lin, M.; Xu, X.; Tang, X.; Zhao, Y.; Xu, H. pH-Sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Chen, L.; Qu, Y.; Peng, J.; Chu, B.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.; Qian, Z. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-Irannejad, V.; Samadi, N.; Salehi, R.; Yousefi, B.; Rahimi, M.; Akbarzadeh, A.; Zarghami, N. Reversion of Multidrug Resistance by Co-Encapsulation of Doxorubicin and Metformin in Poly (lactide-co-glycolide)-d-α-tocopheryl Polyethylene Glycol 1000 Succinate Nanoparticles. Pharm. Res. 2018, 35, 119. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Xie, F.; Lu, Y.; Yin, C.; Shen, X. Co-Delivery of Docetaxel and Salinomycin to Target Both Breast Cancer Cells and Stem Cells by PLGA/TPGS Nanoparticles. Int. J. Nanomed. 2019, 14, 9199–9216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Chen, Q.; Qi, Y.; Liu, Z.; Hao, T.; Sun, X.; Qiao, M.; Ma, X.; Xu, T.; Zhao, X.; et al. Rational Design of Multifunctional Polymeric Nanoparticles Based on Poly(l-histidine) and d-α-Vitamin E Succinate for Reversing Tumor Multidrug Resistance. Biomacromolecules 2018, 19, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tao, W.; Zhang, H.; Liu, G.; Wang, T.; Zhang, L.; Zeng, X.; Mei, L. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 2016, 30, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fu, S.; Peng, Q.; Han, Y.; Xie, J.; Zan, N.; Chen, Y.; Fan, J. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: In vitro and in vivo evaluation. Int. J. Pharm. 2017, 516, 313–322. [Google Scholar] [CrossRef]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2423. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.; Wang, T.; Dong, G.; Wu, S.H.; Young, T.; Shieh, M.; Lou, P.; Lin, F. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 2018, 28, 3996–4005. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Wu, S.Y.-H.; Wang, W.-H.; Peng, C.-L.; Lin, F.-H.; Lin, C.; Young, T.-H.; Shieh, M.J. Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials 2008, 29, 3014–3022. [Google Scholar] [CrossRef]
- Tseng, C.; Su, W.; Yen, K.; Yang, K.; Lin, F. The use of biotinylated-EGFmodified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef]
- Choi, S.; Byeon, H.; Choi, J.; Thao, L.; Kim, I.; Lee, E.; Shin, B.; Lee, K.; Youn, Y. Inhalable self-assembled albumin nanoparticles for treating drug resistant lung cancer. J. Control. Release 2015, 197, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Murdter, T.; Philippi, C.; Loretz, B.; Schaefer, U.; Lehr, C.; Schwab, M.; Ammon-Treiber, S. Pulmonary delivery and tissue distribution of aerosolized antisense 2′-O-Methyl RNA containing nanoplexes in the isolated perfused and ventilated rat lung. Eur. J. Pharm. Biopharm. 2012, 81, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Schneider, M.; Friebel, K.; Dong, M.; Schaefer, U.; Murdter, T.; Lehr, C. Treatment of lung cancer via telomerase inhibition: Self-assembled nanoplexes versus polymeric nanoparticles as vectors for 2′-O-Methyl-RNA. Eur. J. Pharm. Biopharm. 2012, 80, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallak, K.M.; Azarmi, S.; Anwar-Mohamed, A.; Roa, W.H.; Löbenberg, R. Secondary cytotoxicity mediated by alveolar macrophages: A contribution to the total efficacy of nanoparticles in lung cancer therapy? Eur. J. Pharm. Biopharm. 2010, 76, 112–119. [Google Scholar] [CrossRef]
- Zhong, Q.; da Rocha, S.R. Poly (amidoamine) Dendrimer-Doxorubicin Conjugates: In Vitro Characteristics and Pseudosolution Formulation in Pressurized Metered-Dose Inhalers. Mol. Pharm. 2016, 13, 1058–1072. [Google Scholar] [CrossRef]
- Xie, Y.; Aillon, K.L.; Cai, S.; Christian, J.M.; Davies, N.M.; Berkland, C.J.; Forrest, M.L. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int. J. Pharm. 2010, 392, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Gautam, A.; Densmore, C.L.; Melton, S.; Golunski, E.; Waldrep, J.C. Aerosol delivery of PEI-p53 complexes inhibits B16-F10 lung metastases through regulation of angiogenesis. Cancer Gene Ther. 2002, 9, 28–36. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Jiang, H.; Kim, J.; Lee, A.; Kim, S.; Cho, C.; Cho, M. Dual expression of shAkt1 and Pdcd4 suppresses lung tumorigenesis in K-rasLA1 mice. Anticancer Res. 2015, 35, 2015–2019. [Google Scholar]
- Kim, H.W.; Park, I.K.; Cho, C.S.; Lee, K.H.; Beck, G.R.; Colburn, N.H.; Cho, M.H. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004, 64, 7971–7976. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Kim, T.H.; Hwang, S.K.; Chang, S.H.; Kim, H.W.; Anderson, H.K.; Lee, H.W.; Lee, K.H.; Colburn, N.H.; Yang, H.S.; et al. Aerosol delivery of urocanic acid-modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K-ras null mice. Mol. Cancer Ther. 2006, 5, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Xu, C.X.; Kim, H.W.; Chung, Y.S.; Shin, J.Y.; Chang, S.H.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; et al. Urocanic acid-modified chitosan-mediated PTEN delivery via aerosol suppressed lung tumorigenesis in K-ras(LA1) mice. Cancer Gene Ther. 2008, 15, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.L.; Hong, S.H.; Kim, Y.K.; Islam, M.A.; Kim, H.J.; Choi, Y.J.; Nah, J.W.; Lee, K.H.; Han, K.W.; Chae, C.; et al. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int. J. Pharm. 2011, 420, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Cho, C.S.; Cho, M.H.; Jiang, H.L. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J. Biomed. Mater Res. A 2014, 102, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Kim, Y.K.; Arote, R.; Nah, J.W.; Cho, M.H.; Choi, Y.J.; Akaike, T.; Cho, C.S. Chitosan-graft-polyethylenimine as a gene carrier. J. Control. Release 2007, 117, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Xu, C.X.; Kim, Y.K.; Arote, R.; Jere, D.; Lim, H.T.; Cho, M.H.; Cho, C.S. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials 2009, 30, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Tornos, C.; Qiu, X.; Lia, M.; Perez-Soler, R. p53 Aerosol formulation with low toxicity and high efficiency for early lung cancer treatment. Clin. Cancer Res. 2007, 13, 4900–4908. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.X.; Jere, D.; Jin, H.; Chang, S.H.; Chung, Y.S.; Shin, J.Y.; Kim, J.E.; Park, S.J.; Lee, Y.H.; Chae, C.H.; et al. Poly(ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesis. Am. J. Respir. Crit. Care Med. 2008, 178, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, P.; Zhang, J.; Tian, H.; Park, K.; Chen, X. Pulmonary Codelivery of Doxorubicin and siRNA by pH-Sensitive Nanoparticles for Therapy of Metastatic Lung Cancer. Small 2015, 11, 4321–4333. [Google Scholar] [CrossRef]
- Xu, C.; Tian, H.; Sun, H.; Jiao, Z.; Zhang, Y.; Chen, X. A pH sensitive co-delivery system of siRNA and doxorubicin for pulmonary administration to B16F10 metastatic lung cancer. RSC Adv. 2015, 5, 103380–103385. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, H.; Huang, L. Smart polymeric nanoparticles for cancer gene delivery. Mol. Pharm. 2015, 12, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, T.; Pringsheim, T. Nutraceuticals in Migraine: A Summary of Existing Guidelines for Use. Headache 2016, 56, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Clin. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivellese, A.A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The Possible Role of Nutraceuticals in the Prevention of Cardiovascular Disease. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2019, 26, 101–111. [Google Scholar] [CrossRef]
- Poli, A.; Visioli, F. Pharmacology of Nutraceuticals with Lipid Lowering Properties. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2019, 26, 113–118. [Google Scholar] [CrossRef]
- Borghi, C.; Cicero, A.F.G. Nutraceuticals with a clinically detectable blood pressure-lowering effect: A review of available randomized clinical trials and their meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; Group, P. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef] [Green Version]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results from a Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef]
- Varela-López, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. Nutraceuticals in Periodontal Health: A Systematic Review on the Role of Vitamins in Periodontal Health Maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef] [Green Version]
- Orr, S.L. The Evidence for the Role of Nutraceuticals in the Management of Pediatric Migraine: A Review. Curr. Pain Headache Rep. 2018, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Leong, D.J.; Cardoso, L.; Sun, H.B. Nutraceuticals and osteoarthritis pain. Pharmacol. Ther. 2018, 187, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Caballero, S.; Davidov-Pardo, G. Bioavailability of nanotechnology-based bioactives and nutraceuticals. Adv. Food Nutr. Res. 2019, 88, 235–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P.; Sinclair, D.A.; Bonkowski, M.S.; Coleville, A.J.; Powell, D.; et al. Resveratrol Improves Vascular Function and Mitochondrial Number but Not Glucose Metabolism in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wu, X.; Dong, W.; Sun, W.; Li, J.; Tang, X. Enhancement by sodium caprate and sodium deoxycholate of the gastrointestinal absorption of berberine chloride in rats. Drug Dev. Ind. Pharm. 2013, 39, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piskula, M.K.; Murota, K.; Terao, J. Bioavailability of flavonols and flavones. In Flavonoids and Related Compounds: Bioavailability and Function; CRC Press: Boca Raton, FL, USA, 2012; pp. 93–107. [Google Scholar]
- Rich, G.T.; Buchweitz, M.; Winterbone, M.S.; Kroon, P.A.; Wilde, P.J. Towards an Understanding of the Low Bioavailability of Quercetin: A Study of Its Interaction with Intestinal Lipids. Nutrients 2017, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, A.; Mukai, R.; Sakao, K.; Terao, J.; Hou, D.-X. Anti-inflammatory effects and molecular mechanisms of 8-prenyl quercetin. Mol. Nutr. Food Res. 2016, 60, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Barahona, M.J.; Baratta, V.; Ollodart, J.; Mulligan, D.; Geibel, J.P. Design and implementation of novel nutraceuticals and derivatives for treating intestinal disorders. Future Med. Chem. 2019, 11, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ren, C.; Li, J.; Li, B. Enhancing the photostability and bioaccessibility of resveratrol using ovalbumin-carboxymethylcellulose nanocomplexes and nanoparticles. Food Funct. 2018, 9, 3788–3797. [Google Scholar] [CrossRef] [PubMed]
- Jaisamut, P.; Wiwattanawongsa, K.; Wiwattanapatapee, R. A Novel Self-Microemulsifying System for the Simultaneous Delivery and Enhanced Oral Absorption of Curcumin and Resveratrol. Planta Med. 2017, 83, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, P.; Bandyopadhyay, A.; Chakraborty, A.; Sarkar, K. Enhancement of anticancer activity and drug delivery of chitosan-curcumin nanoparticle via molecular docking and simulation analysis. Carbohydr. Polym. 2018, 182, 188–198. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Co-delivery of curcumin and serratiopeptidase in HeLa and MCF-7 cells through nanoparticles show improved anti-cancer activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 673–684. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, X.; Shen, M.; Ru, D.; Gao, P.; Zhang, X.; Huang, C.; Sun, Y.; Li, H.; Duan, Y. Co-Delivery of Triptolide and Curcumin for Ovarian Cancer Targeting Therapy via mPEG-DPPE/CaP Nanoparticle. J. Biomed. Nanotechnol. 2018, 14, 1761–1772. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination Therapy for Ulcerative Colitis: Orally Targeted Nanoparticles Prevent Mucosal Damage and Relieve Inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef]
- Gugulothu, D.; Kulkarni, A.; Patravale, V.; Dandekar, P. pH-sensitive nanoparticles of curcumin-celecoxib combination: Evaluating drug synergy in ulcerative colitis model. J. Pharm. Sci. 2014, 103, 687–696. [Google Scholar] [CrossRef]
- Niazvand, F.; Khorsandi, L.; Abbaspour, M.; Orazizadeh, M.; Varaa, N.; Maghzi, M.; Ahmadi, K. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on mono-iodoacetate -induced osteoarthritis in rats. Vet. Res. Forum Int. Q. J. 2017, 8, 155–161. [Google Scholar]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [Green Version]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-encapsulated resveratrol and quercetin in chitosan and peg modified chitosan nanoparticles: For efficient intra ocular pressure reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shi, Y.; Han, Q.; Dai, G. Endothelial growth factor receptor-targeted and reactive oxygen species-responsive lung cancer therapy by docetaxel and resveratrol encapsulated lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2018, 105, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, 13, 4133–4144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Sójka, M.; Klewicka, E.; Lipińska, L.; Klewicki, R.; Kołodziejczyk, K. Ellagitannins from Rubus idaeus L. Exert Geno- and Cytotoxic Effects against Human Colon Adenocarcinoma Cell Line Caco-2. J. Agric. food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Toxicity Potential of Nutraceuticals. Methods Mol. Biol. 2018, 1800, 367–394. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Liu, L.; Gong, Y.; Xie, Y.; Cao, Y. Chemical Structures of Polyphenols That Critically Influence the Toxicity of ZnO Nanoparticles. J. Agric. Food Chem. 2018, 66, 1714–1722. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, R.; McClements, D.J.; Chefetz, B.; Polubesova, T.; Xing, B. Transformation and Speciation Analysis of Silver Nanoparticles of Dietary Supplement in Simulated Human Gastrointestinal Tract. Environ. Sci. Technol. 2018, 52, 8792–8800. [Google Scholar] [CrossRef]
- Vozza, G.; Khalid, M.; Byrne, H.J.; Ryan, S.M.; Frias, J.M. Nutraceutical formulation, characterisation, and in-vitro evaluation of methylselenocysteine and selenocystine using food derived chitosan:zein nanoparticles. Food Res. Int. 2019, 120, 295–304. [Google Scholar] [CrossRef]
- Alphandéry, E.; Grand-Dewyse, P.; Lefèvre, R.; Mandawala, C.; Durand-Dubief, M. Cancer therapy using nanoformulated substances: Scientific, regulatory and financial aspects. Expert Rev. Anticancer Ther. 2015, 15, 1233–1255. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Fattal, E.; Nicolas, J. From poly(alkyl cyanoacrylate) to squalene as core material for the design of nanomedicines. J. Drug Target. 2019, 27, 470–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledford, H. Bankruptcy filing worries developers of nanoparticle cancer drugs. Nature 2016, 533, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Writer, S. Onxeo’s liver cancer drug Livatag fails in phase 3 trial. In Pharmaceutical Business Review; 2017; Volume 2017, Available online: https://pharmaceutical-business-review.com/ (accessed on 13 June 2020).
- Rodríguez Fernández, C. French Nanoparticle Therapy Fails to Improv Liver Cancer Treatment in Phase III. Available online: https://www.labiotech.eu/medical/onxeo-livatag-nanoparticle-cancer/ (accessed on 13 June 2020).






| Type of Nanoparticle | Nanoparticle Composition | Drug Delivery | Treatment | Reference |
|---|---|---|---|---|
| Polymeric Micelles | PLGA/PVA | bevacizumab | Choroidal and retinal neovascularization | [33] |
| dexamethasone | Ocular inflammation | [34] | ||
| fenofibrate | Retinal dysfunctions, retinal leukostasis, retinal vascular leakage, over expression of VEGF, choroidal neovascularization | [35] | ||
| PLGA/PVA/PEI | bevacizumab and dexamethasone | Choroidal neovascularization | [36] | |
| PLGA/Tween 80, poloxamer 188 or Brij® | brinzolamide | Ocular pressure | [37] | |
| PLGA/Pluronic F127 | dexamethasone | Immunologic graft rejection | [38] | |
| PLGA/PVP | bevacizumab | Age-related macular degeneration | [39] | |
| CH/Sodium tripolyphosphate | levofloxacin | Ocular infections | [40] | |
| bevacizumab | Choroidal neovascularization | [41] | ||
| CH/Sodium tripolyphosphate/hyaluronic acid | ceftazidime | Ocular infections | [42] | |
| CH/PVA/sodium deoxycholate | prednisolone | Ocular inflammation | [43] | |
| Stearic acid and valylvaline functionalized CH | dexamethasone | Ocular inflammation, retinal dysfunctions, retinal leukostasis, retinal vascular leakage, over expression of VEGF, choroidal neovascularization | [44] | |
| Cationic CH grafted methoxy poly(ethylene glycol)-poly(ε-caprolactone) | diclofenac | Ocular inflammation | [45] | |
| Methoxy poly(ethylene glycol)-poly(lactide) block copolymer | cyclosporine A | Dry eye syndrome | [46] | |
| Tween80/polyoxyethylene stearate | everolimus | Autoimmune uveoretinitis, non-infectious uveitis, corneal neovascularization and immune-mediated rejection | [47] | |
| PVA/Poloxamer P407/hydroxypropyl methylcellulose | ||||
| PEG–PCL–PEG | triamcinolone acetonide | Ocular inflammation | [48] | |
| Lecithin-based NPs embedded in poloxamers gel (P188 and P407) | dexamethasone | [49] | ||
| PLGA–PEG NPs embedded in PEG–PLGA–PEG gel | triamcinolone acetonide | Age-related macular degeneration | [50] | |
| Bevacizumab-coated PLA NPs embedded in PLGA microparticles | bevacizumab | [51] | ||
| Dendrimeric nanocarriers | PEGylated polyamidoamine modified with cyclic arginine–glycine–aspartate hexapeptide and penetration | – | Posterior ocular diseases | [52] |
| Timolol-derivatized polyamidoamine | timolol | Ocular hypertension | [53] | |
| Polyamidoamine/hyaluronic acid | antisense oligonucleotides | Regulation of the expression of target proteins and genes in cells | [54] | |
| Cyclodextrins | Propylamino-β-Cyclodextrin | latanoprost | Glaucoma | [60] |
| γ-Cyclodextrin and randomly methylated β-cyclodextrin | celecoxib | Age-related macular degeneration and diabetic retinopathy | [61,62] | |
| α-Cyclodextrin/Soluplus/Pluronic P103 | natamycin | Fungal keratitis | [63] | |
| Polymeric vesicles | DOTAP/DOPE/DSPE–PEG | siRNA sequences/chlorhexidine | Keratitis caused by Acanthamoeba | [65] |
| Precirol® ATO 5/castor oil/Span® 80/mPEG-2K-DSPE | natamycin | Fungal keratitis | [66] |
| Polymer | Active Principle | Type of Cancer | Experimental Model/Route | Size (nm) | Z Potential (mV) | PDI | References |
|---|---|---|---|---|---|---|---|
| PEGylated PLGA | doxorubicin | various | In vivo: Bioavailability assay in Wistar rat Oral | 183.10 | −13.10 | 0.132 | [136] |
| PACA | doxorubicin–cyclosporin A. | various | In vitro: P388/ADR cells line | 288 | * | * | [137] |
| Lip–BSA | paclitaxel | various | In vivo: 4T1 cells in BALB/c mice Tail vein | 116.2 | −18.4 | 0.307 | [138] |
| PCL–PEG | camptothecin | glioma | In vivo: 4T1 cells in BALB/c mice Tail vein | 274 | −19 | 0.07 | [139] |
| PLGA–PEG | paclitaxel | glioma | In vivo: gliosarcoma 9L cells in Fischer F344 rats Direct injection | 121 | 23.7 | 0.088 | [140] |
| PLGA–Cyanine5.5 | doxorubicin | glioblastoma | In vivo: C6 Glioma cells in Wistar rats and nude mice Tail vein | 114 | −14.9 | 0.196 | [141] |
| PBCA | doxorubicin | glioblastoma | In vitro: U87 glioblastoma human cells line | 260 | −19 | 0.02 | [142] |
| PLA–PEG–maleimide | paclitaxel | breast cancer (TNB) | In vitro: MDA-MB-231 cells In vivo: BALB/c homozygous nude mice Intravenous injection. Tail vein | 212 | −16.34 | 0.183 | [143] |
| mPEG–PLGA–PGlu | doxorubicin–curcumin | breast cancer | In vivo: LM2 cells in BALB/c homozygous nude mice Tail vein | 107.5 | −13.7 | * | [144] |
| PCLLA–PEG–PCLLA | doxorubicin and Chlorin e6-MnO2 | breast cancer | In vivo: MCF-7/ADR cells xenograft in female BALB/c nude mice. Tail vein | 120 | −8.9 | * | [145] |
| TPGS–PLGA | doxorubicin and metformin | breast cancer | In vivo: MCF-7 cells in nude mice Tail vein | 87 | −3.5 | 0.5 | [146] |
| TPGS–PLGA | docetaxel and salinomycin | breast cancer | In vitro: MCF-7/DOX cell line | 73.83 | −25.7 | 0.193 | [147] |
| Gal–pD–TPGS–PLA | docetaxel | liver cancer | In vivo: MCF-7 cells in BALB/c mice Orthotopic injection | 209.4 | 13.7 | 0.145 | [149] |
| PCL–PEGPEG–PCL | paclitaxel | lung cancer | In vivo: MCF-7/ADR cells in BALB/c nude mice Intravenous injection | 168 | −12.49 | 0.19 | [150] |
| PEI–PLA | paclitaxel | lung cancer | In vivo: A549 cells in BALB/c mice. Tail vein | 67.31 | 30.3 | 0.105 | [151] |
| Polymeric Nanocarrier | Active Principle | Preparation Method | Size (nm) | Z Potential (mV) | PDI | References |
|---|---|---|---|---|---|---|
| Gelatin NPs | cisplatin | desolvation | 220 | −9.3 | 0.287 | [152,153,154] |
| HSA NPs | doxorubicin + TRAIL | self-assembly | 341.6 | * | * | [155] |
| CH/PLGA NPs | OMR | emulsion–diffusion | 160 | 29 | 0.033 | [156,157] |
| BIPCA NPs | doxorubicin | emulsion polymerization | 137.2 | 23.5 | 0.12 | [158] |
| PEGylated PAMAM dendrimers | doxorubicin | chemical conjugation | 26.1 | −6.6 | 0.108 | [159] |
| Hyaluronan conjugates | cisplatin | covalent bonding | * | * | * | [160] |
| PEI polyplexes | p53 | electrostatic complexation | * | * | * | [161] |
| SDA–PEI polyplexes | PDCD4 + shAkt1 | electrostatic complexation | * | * | * | [162] |
| Glucosylated PEI polyplexes | PTEN | electrostatic complexation | * | * | * | [163] |
| UACH polyplexes | PDCD4 PTEN | electrostatic complexation | * | * | * | [164,165] |
| SPE–GPT polyplexes | shAkt1 | electrostatic complexation | 163.2 | 9.14 | 0.192 | [166] |
| SPE–PEG polyplexes | PDCD4 | electrostatic complexation | 130 | 8.61 | 1.13 | [167] |
| CH–g–PEI polyplexes | shAkt1 | electrostatic complexation | 166.4 | −20 | * | [168,169] |
| PLL/protamine polyplexes | p53 | electrostatic complexation | * | * | * | [170] |
| PEI–alt–PEG polyplexes | Akt1 siRNA | electrostatic complexation | * | * | * | [171] |
| PEI polyplexes | doxorubicin + Bcl2 siRNA | electrostatic complexation | 78.2 | 20.4 | * | [172,173] |
| Drug Delivery | Polymeric Nanoparticle | Experimental Model/Route | Results | Reference |
|---|---|---|---|---|
| CD98 siRNA plus curcumin | HA-functionalized NP encapsulated in hydrogel (CH: alginate; 3:7) | In vitro: Caco2-BBE and Raw 2647 cells | ↑Cellular uptake ↓Expressions of CD98 and TNF-α | [203] |
| In vivo: DSS-induced UC/orally | ↓Weight loss ↓Fecal Lcn-2 levels ↓MPO activity ↓Histological damage ↓CD98 and TNF-α mRNA expression | |||
| curcumin plus celecoxib | pH sensitive enteric polymer NP (Eudragit® S100) | In vivo: TNBS-induced UC/orally | ↓MPO, SOD and LPO ↓Leukocyte infiltration | [204] |
| curcumin | Biopolymeric CH NP | In vitro: HeLa cells | ↓Proliferation and viability cell ↑Apoptotic activity, DNA damage, cell-cycle blockage and ROS levels | [200] |
| curcumin | PLGA NP | In vivo: MIA-induced OA/orally | ↑Cellularity and matrix | [205] |
| curcumin | Theracurmin® NP | In vivo: DSS-induced UC/orally | ↓NF-κB, TNF-α, IL-1β, IL-6, CXCL1 and CXCL2 and neutrophil infiltration ↑CD4+ and Foxp3+ T cells ↑CD103+ and CD8α− dendritic cells ↑Clostridium cluster IV and XIVa ↑Butyrate levels (bacteria and fecal) | [206] |
| resveratrol plus quercetin | PEG modified CH NP | Ex-vivo: Albino rabbit cornea | ↑Solubility and permeation ↓Intraocular pressure | [207] |
| docetaxel plus resveratrol | EGF conjugated core-shell lipid–polymer hybrid NP | In vitro: HCC827, NCIH2135 and HUVEC cells | ↓Tumoral cell viability | [208] |
| In vivo: lung cancer animal model/intravenously | ↓Body weight loss ↓Tumor volume ↑Tumor growth inhibition | |||
| resveratrol | Galactosylated NP (NP combined with a ligand (galactose) for improved route of intestinal transport by the way of SGLT1) | In vitro: Raw 2647 cells | ↓TNF-α, IL-6 and NO | [209] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. https://doi.org/10.3390/nano10071403
Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, Alcudia A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials. 2020; 10(7):1403. https://doi.org/10.3390/nano10071403
Chicago/Turabian StyleBegines, Belén, Tamara Ortiz, María Pérez-Aranda, Guillermo Martínez, Manuel Merinero, Federico Argüelles-Arias, and Ana Alcudia. 2020. "Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects" Nanomaterials 10, no. 7: 1403. https://doi.org/10.3390/nano10071403
APA StyleBegines, B., Ortiz, T., Pérez-Aranda, M., Martínez, G., Merinero, M., Argüelles-Arias, F., & Alcudia, A. (2020). Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials, 10(7), 1403. https://doi.org/10.3390/nano10071403