Disposable Stochastic Sensors Based on Nanolayer Deposition(s) of Silver and AgC Composite on Plastic for the Assay of α-amylase in Whole Blood and Saliva
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
2.3. Design of Disposable Stochastic Sensors
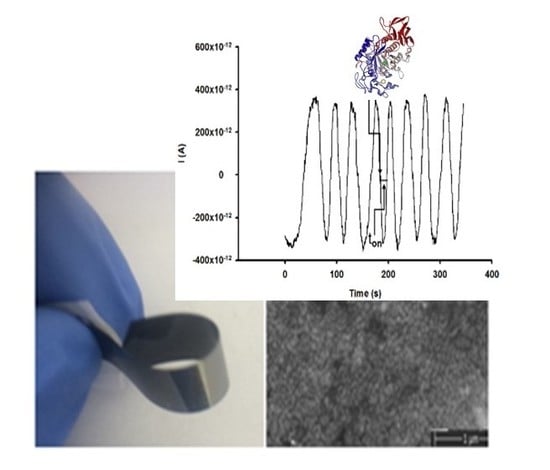
2.4. Stochastic Mode
2.5. Samples
3. Results and Discussions
3.1. Response Characteristics of the Stochastic Sensor Used for the Determination of Pancreatic (PAA) and Salivary (SAA) α-Amylase
3.2. Selectivity of the Stochastic Sensors
3.3. Analytical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Pieper-Bigelow, C.; Strocchi, A.; Levitt, M.D. Where does serum amylase come from and where does it go? Gastroenterol. Clin. N. Am. 1990, 19, 793–810. [Google Scholar] [PubMed]
- Zakowski, J.J.; Bruns, D.E. Biochemistry of human alpha amylase isoenzymes. Crit. Rev. Clin. Lab. Sci. 1985, 21, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Panteghini, M.; Ceriotti, F.; Pagani, F.; Secchiero, S.; Zaninotto, M.; Franzini, C. Recommendations for the routine use of pancreatic amylase. Measurement instead of total amylase for the diagnosis and monitoring of pancreatic pathology. Clin. Chem. Lab. Med. 2002, 40, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Miao, P. Electrochemical Analysis of Proteins and Cells; Springer: Berlin, Germany, 2013. [Google Scholar]
- Zhang, X.; Guo, Q.; Cui, D. Recent Advances in Nanotechnology Applied to Biosensors. Sensor 2009, 9, 1033–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wang, J.; Xu, Y.; Li, G. Combination of enzyme catalysis and electrocatalysis for biosensor fabrication: Application to assay the activity of indoleamine 2,3-dioxygensae. Biosens. Bioelectron. 2010, 26, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Usui, T.; Kaneda, Y.; Ikeda, T. Amylase assay in human serum using an oligosaccharide dehydrogenase-modified graphite paste electrode containing benzoquinone, J-Stage. Bunseki Kagaku 1992, 41, T145–T149. [Google Scholar] [CrossRef] [Green Version]
- Zajoncova, L.; Jilek, M.; Beranova, V.; Pec, P. A biosensor for the determination of amylase activity. Biosens. Bioelectron. 2004, 20, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Ilie-Mihai, R.M.; Gugoasa, L.A.; Bilasco, A.; Visan, C.A.; Streinu-Cercel, A. Molecular recognition of IL-8, IL-10, IL-12, and IL-15 in biological fluids using phthalocyanine-based stochastic sensors. Anal. Bioanal. Chem. 2018, 410, 7723–7737. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Popa-Tudor, I.; Ionescu-Tirgoviste, C.; Stoica, R.A. Molecular recognition of pyruvic acid and L-lactate in early-diabetic stage. J. Electrochem. Soc. 2018, 165, B659–B664. [Google Scholar] [CrossRef]
- Gugoasa, L.A.; Stefan-van Staden, R.I.; Dima, A.; Visan, C.A.; Streinu-Cercel, A.; Biris, A.; Calenic, B. Fast screening of biological fluids for cytokines and adipokines using stochastic sensing. Microelectron. Eng. 2015, 148, 64–69. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwal, S.S.; Zhang, Q.; Sun, C.; Thakur, V.K. Graphitic carbon nitride doped copper–manganese alloy as high–performance electrode material in supercapacitor for energy storage. Nanomaterials 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan-van Staden, R.I.; Ilie-Mihai, R.M.; Magerusan, L.; Coros, M.; Pruneanu, S. Enantioanalysis of glutamine—A key factor in establishing the metabolomics process in gastric cancer. Anal. Bioanal. Chem. 2020, 412, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Cioates Negut, C.; Stefan–van Staden, R.I.; Harja, F.; van Staden, J.F. Pattern recognition of amino acids in wines. Electroanalysis 2020, 32, 7–10. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I.; Balahura, L.R.; Cioates-Negut, C.; Aboul-Enein, H.Y. Stochastic microsensors used for the assessment of DNA damage in leukemia. Anal. Biochem. 2020, 605, 113839. [Google Scholar] [CrossRef] [PubMed]







| Microsensors Based On | Calibration Equation and Correlation Coefficient (r) * | Linear Concentration Range (U mL−1) | toff (s) | Sensitivity (s−1/U mL−1) | Limit of Determination (U mL−1) |
|---|---|---|---|---|---|
| α-CD/Ag | 1/ton = 0.02 + 2.49(± 0.05) × 103 × C r = 0.9993 | 1.00 × 10−7–1.00 × 103 | 0.6 ± 0.1 | 2.49 × 103 | 1.00 × 10−7 |
| α-CD/AgC | 1/ton =0.03 + 1.85(± 0.07) × 103 × C r = 0.9999 | 1.00 × 10−7–1.00 × 103 | 1.2 ± 0.2 | 1.85 × 103 | 1.00 × 10−7 |
| α-CD/AgC/Ag | 1/ton =0.03 + 1.72(± 0.02) × 103 × C r = 0.9999 | 1.00 × 10−7–1.00 × 103 | 1.2 ± 0.1 | 1.72 × 103 | 1.00 × 10−7 |
| Microsensors Based On | Calibration Equation and Correlation Coefficient (r) * | Linear Concentration Range (U mL−1) | toff (s) | Sensitivity (s−1/U mL−1) | Limit of Determination (U mL−1) |
|---|---|---|---|---|---|
| α-CD/Ag | 1/ton = 0.01 + 7.07(± 0.05) × 108 × C r = 0.9994 | 1.5 × 10−9–1.5 × 102 | 0.6 ± 0.1 | 7.07 × 108 | 1.5 × 10−9 |
| α-CD/AgC | 1/ton =0.01 + 3.10(± 0.05) × 106 × C r = 0.9013 | 1.5 × 10−15–1.5 × 102 | 1.0 ± 0.2 | 3.10 × 106 | 1.5 × 10−15 |
| α-CD/AgC/Ag | 1/ton = 0.01 + 1.20(± 0.05) × 107 × C r = 0.9704 | 1.5 × 10−15–1.5 × 102 | 0.5 ± 0.1 | 1.20 × 107 | 1.5 × 10−15 |
| Microsensors Based On | %, Recovery of PAA |
|---|---|
| α-CD/Ag | 99.78 ± 0.03 |
| α-CD/AgC | 99.73 ± 0.05 |
| α-CD/AgC/Ag | 99.85 ± 0.02 |
| Microsensors Based On | %, Recovery of SAA |
|---|---|
| α-CD/Ag | 99.85 ± 0.05 |
| α-CD/AgC | 99.80 ± 0.03 |
| α-CD/AgC/Ag | 99.98 ± 0.02 |
| Microsensors Based On | PAA, U L−1 | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| α-CD/Ag | 43.77 ± 0.05 | 23.13 ± 0.04 | 27.35 ± 0.02 | 42.80 ± 0.04 | 80.01 ± 0.05 |
| α-CD/AgC | 43.14 ± 0.07 | 24.21 ± 0.04 | 28.49 ± 0.02 | 43.77 ± 0.05 | 80.52 ± 0.03 |
| α-CD/AgC/Ag | 42.14 ± 0.03 | 23.87 ± 0.03 | 28.53 ± 0.04 | 42.61 ± 0.02 | 80.42 ± 0.02 |
| Microsensors Based On | SAA, U mL−1 | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| α-CD/Ag | 86.78 ± 0.03 | 32.70 ± 0.03 | 59.90 ± 0.05 | 79.88 ± 0.03 | 53.77 ± 0.03 |
| α-CD/AgC | 86.30 ± 0.04 | 32.88 ± 0.05 | 59.99 ± 0.05 | 79.23 ± 0.03 | 53.58 ± 0.04 |
| α-CD/AgC/Ag | 86.90 ± 0.05 | 32.87 ± 0.01 | 59.55 ± 0.02 | 79.33 ± 0.04 | 54.05 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan-van Staden, R.-I.; Moscalu-Lungu, A.; Badulescu, M. Disposable Stochastic Sensors Based on Nanolayer Deposition(s) of Silver and AgC Composite on Plastic for the Assay of α-amylase in Whole Blood and Saliva. Nanomaterials 2020, 10, 1528. https://doi.org/10.3390/nano10081528
Stefan-van Staden R-I, Moscalu-Lungu A, Badulescu M. Disposable Stochastic Sensors Based on Nanolayer Deposition(s) of Silver and AgC Composite on Plastic for the Assay of α-amylase in Whole Blood and Saliva. Nanomaterials. 2020; 10(8):1528. https://doi.org/10.3390/nano10081528
Chicago/Turabian StyleStefan-van Staden, Raluca-Ioana, Alexandrina Moscalu-Lungu, and Marius Badulescu. 2020. "Disposable Stochastic Sensors Based on Nanolayer Deposition(s) of Silver and AgC Composite on Plastic for the Assay of α-amylase in Whole Blood and Saliva" Nanomaterials 10, no. 8: 1528. https://doi.org/10.3390/nano10081528
APA StyleStefan-van Staden, R. -I., Moscalu-Lungu, A., & Badulescu, M. (2020). Disposable Stochastic Sensors Based on Nanolayer Deposition(s) of Silver and AgC Composite on Plastic for the Assay of α-amylase in Whole Blood and Saliva. Nanomaterials, 10(8), 1528. https://doi.org/10.3390/nano10081528