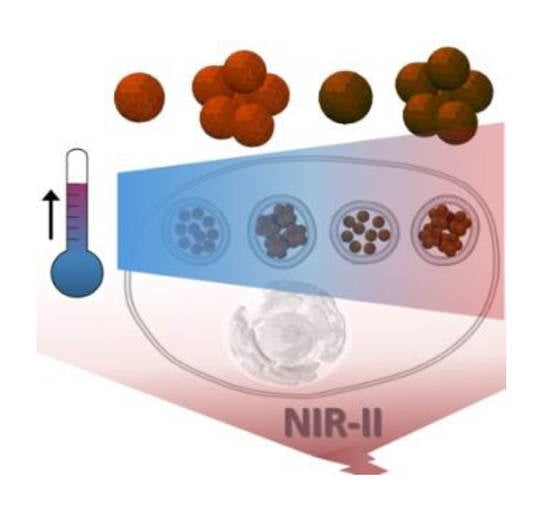
Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Iron Oxide Nanoparticles
2.3. Elemental Analysis
2.4. UV-Vis-NIR-I-II Spectroscopy
2.5. Magnetic Hyperthermia and Photothermia Measurements
2.6. Cell Culture and Internalization Assays
2.7. Cytotoxicity Study
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, L.; Hou, Y.; Jiao, M.; Qiao, R.; Jia, Q.; Liu, C.; Fang, F.; Lei, H.; Gao, M. Anchoring group effects of surface ligands on magnetic properties of Fe3O4 nanoparticles: Towards high performance MRI contrast agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [Green Version]
- Van de Walle, A.; Perez, J.E.; Abou-Hassan, A.; Hémadi, M.; Luciani, N.; Wilhelm, C. Magnetic nanoparticles in regenerative medicine: What of their fate and impact in stem cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]
- Mary, G.; Van de Walle, A.; Perez, J.E.; Ukai, T.; Maekawa, T.; Luciani, N.; Wilhelm, C. High-throughput differentiation of embryonic stem cells into cardiomyocytes with a microfabricated magnetic pattern and cyclic stimulation. Adv. Funct. Mater. 2020, 30, 2002541. [Google Scholar] [CrossRef]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems based on magnetic nanoparticles and Thermo- or pH-Responsive polymers: An update and future perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.-S.; Wilhelm, C.; Lesieur, S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials 2008, 29, 4137–4145. [Google Scholar] [CrossRef] [PubMed]
- Derec, C.; Wilhelm, C.; Servais, J.; Bacri, J.-C. Local control of magnetic objects in microfluidic channels. Microfluid. Nanofluidics 2009, 8, 123. [Google Scholar] [CrossRef]
- Ferraro, D.; Lin, Y.; Teste, B.; Talbot, D.; Malaquin, L.; Descroix, S.; Abou-Hassan, A. Continuous chemical operations and modifications on magnetic γ-Fe2O3 nanoparticles confined in nanoliter droplets for the assembly of fluorescent and magnetic SiO2@γ-Fe2O3. Chem. Commun. 2015, 51, 16904–16907. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 2019, 138, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; Morales, M.d.P. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef]
- Jonasson, C.; Schaller, V.; Zeng, L.; Olsson, E.; Frandsen, C.; Castro, A.; Nilsson, L.; Bogart, L.K.; Southern, P.; Pankhurst, Q.A.; et al. Modelling the effect of different core sizes and magnetic interactions inside magnetic nanoparticles on hyperthermia performance. J. Magn. Magn. Mater. 2019, 477, 198–202. [Google Scholar] [CrossRef]
- Avugadda, S.K.; Materia, M.E.; Nigmatullin, R.; Cabrera, D.; Marotta, R.; Cabada, T.F.; Marcello, E.; Nitti, S.; Artés-Ibañez, E.J.; Basnett, P.; et al. Esterase-cleavable 2D assemblies of magnetic iron oxide nanocubes: Exploiting enzymatic polymer disassembling to improve magnetic hyperthermia heat losses. Chem. Mater. 2019, 31, 5450–5463. [Google Scholar] [CrossRef]
- Espinosa, A.; Bugnet, M.; Radtke, G.; Neveu, S.; Botton, G.A.; Wilhelm, C.; Abou-Hassan, A. Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia? Nanoscale 2015, 7, 18872–18877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron oxide nanoflowers @ cus hybrids for cancer tri-therapy: Interplay of photothermal therapy, magnetic hyperthermia and photodynamic therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, Y.; Niu, D.; Ma, Z.; Gu, J.; Chen, Y.; Zhao, W.; Liu, X.; Liu, C.; Shi, J. Facile synthesis of monodisperse superparamagnetic Fe3O4 Core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv. Mater. 2011, 23, 5392–5397. [Google Scholar] [CrossRef]
- Melancon, M.P.; Lu, W.; Zhong, M.; Zhou, M.; Liang, G.; Elliott, A.M.; Hazle, J.D.; Myers, J.N.; Li, C.; Jason Stafford, R. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials 2011, 32, 7600–7608. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Yu, T.-J.; Li, P.-H.; Tseng, T.-W.; Chen, Y.-C. Multifunctional Fe3O4/alumina core/shell MNPs as photothermal agents for targeted hyperthermia of nosocomial and antibiotic-resistant bacteria. Nanomedicine 2011, 6, 1353–1363. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, D. Manganese-doped magnetic nanoclusters for hyperthermia and photothermal glioblastoma therapy. ACS Appl. Nano Mater. 2020, 3, 2026–2037. [Google Scholar] [CrossRef]
- Liao, M.-Y.; Lai, P.-S.; Yu, H.-P.; Lin, H.-P.; Huang, C.-C. Innovative ligand-assisted synthesis of NIR-activated iron oxide for cancer theranostics. Chem. Commun. 2012, 48, 5319–5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plan Sangnier, A.; Preveral, S.; Curcio, A.; Silva, A.K.A.; Lefèvre, C.T.; Pignol, D.; Lalatonne, Y.; Wilhelm, C. Targeted thermal therapy with genetically engineered magnetite magnetosomes@RGD: Photothermia is far more efficient than magnetic hyperthermia. J. Control. Release 2018, 279, 271–281. [Google Scholar] [CrossRef]
- Hong, W.-E.; Hsu, I.L.; Huang, S.-Y.; Lee, C.-W.; Ko, H.; Tsai, P.-J.; Shieh, D.-B.; Huang, C.-C. Assembled growth of 3D Fe3O4@Au nanoparticles for efficient photothermal ablation and SERS detection of microorganisms. J. Mater. Chem. B 2018, 6, 5689–5697. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chang, P.-Y.; Liu, C.-L.; Xu, J.-P.; Wu, S.-P.; Kuo, W.-C. New insight on optical and magnetic Fe3O4 nanoclusters promising for near infrared theranostic applications. Nanoscale 2015, 7, 12689–12697. [Google Scholar] [CrossRef] [PubMed]
- Kharey, P.; Dutta, S.B.; Manikandan, M.; Palani, I.A.; Majumder, S.K.; Gupta, S. Green synthesis of near-infrared absorbing eugenate capped iron oxide nanoparticles for photothermal application. Nanotechnology 2019, 31, 095705. [Google Scholar] [CrossRef]
- Van de Walle, A.; Plan Sangnier, A.; Abou-Hassan, A.; Curcio, A.; Hémadi, M.; Menguy, N.; Lalatonne, Y.; Luciani, N.; Wilhelm, C. Biosynthesis of magnetic nanoparticles from nano-degradation products revealed in human stem cells. In Proceedings of the National Academy of Sciences, Urbana, IL, USA, 13 February 2019; pp. 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Lévy, M.; Bacri, J.-C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (hyper) thermia or photothermia? Progressive comparison of iron oxide and gold nanoparticles heating in water, in cells, and in vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Hemery, G.; Keyes, A.C.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning sizes, morphologies, and magnetic properties of monocore versus multicore iron oxide nanoparticles through the controlled addition of water in the polyol synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourinho, F.A.; Franck, R.; Massart, R. Aqueous ferrofluids based on manganese and cobalt ferrites. J. Mater. Sci. 1990, 25, 3249–3254. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Gorski, C.A.; Handler, R.M.; Beard, B.L.; Pasakarnis, T.; Johnson, C.M.; Scherer, M.M. Fe atom exchange between aqueous Fe2+ and magnetite. Environ. Sci. Technol. 2012, 46, 12399–12407. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Myers, M.; Bosnick, K.A.; Brus, L.E. Magnetite Fe3O4 nanocrystals: Spectroscopic observation of aqueous oxidation kinetics†. J. Phys. Chem. B 2003, 107, 7501–7506. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Introduction to the Iron Oxides. In The Iron Oxides; Wiley-VCH GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1–7. [Google Scholar] [CrossRef]
- Lee, N.; Schuck, P.J.; Nico, P.S.; Gilbert, B. Surface enhanced raman spectroscopy of organic molecules on magnetite (Fe3O4) nanoparticles. J. Phys. Chem. Lett. 2015, 6, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Vereda, F.; de Vicente, J.; Morales, M.d.P.; Rull, F.; Hidalgo-Álvarez, R. Synthesis and characterization of single-domain monocrystalline magnetite particles by oxidative aging of Fe(OH)2. J. Phys. Chem. C 2008, 112, 5843–5849. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Maslyakova, G.N.; Chekhonatskaya, M.L.; Terentyuk, G.S.; Navolokin, N.A.; Khlebtsov, B.N.; Khlebtsov, N.G.; Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Plasmonic photothermal therapy: Approaches to advanced strategy. Lasers Surg. Med. 2018, 50, 1025–1033. [Google Scholar] [CrossRef]
- Silva, A.K.; Luciani, N.; Gazeau, F.; Aubertin, K.; Bonneau, S.; Chauvierre, C.; Letourneur, D.; Wilhelm, C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomedicine 2015, 11, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, D.; Coene, A.; Leliaert, J.; Artés-Ibáñez, E.J.; Dupré, L.; Telling, N.D.; Teran, F.J. Dynamical magnetic response of iron oxide nanoparticles inside live cells. ACS Nano 2018, 12, 2741–2752. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. https://doi.org/10.3390/nano10081548
Cabana S, Curcio A, Michel A, Wilhelm C, Abou-Hassan A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials. 2020; 10(8):1548. https://doi.org/10.3390/nano10081548
Chicago/Turabian StyleCabana, Sonia, Alberto Curcio, Aude Michel, Claire Wilhelm, and Ali Abou-Hassan. 2020. "Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers" Nanomaterials 10, no. 8: 1548. https://doi.org/10.3390/nano10081548
APA StyleCabana, S., Curcio, A., Michel, A., Wilhelm, C., & Abou-Hassan, A. (2020). Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials, 10(8), 1548. https://doi.org/10.3390/nano10081548