Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water
Abstract
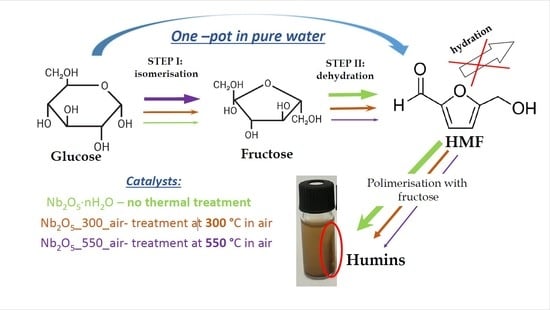
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Catalytic Testing
3. Results and Discussion
3.1. Textural Properties
3.2. Crystallinity of the Samples
3.3. Chemical Properties
3.3.1. X-ray Photoelectron Spectroscopy (XPS) Studies
3.3.2. Acidity of the Catalysts
3.4. Thermogravimetric Analysis
3.5. Catalytic Results
Effects of the Reaction Time and Temperature on the Dehydration of Glucose to 5-Hydroxymethylfurfural (HMF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eblagon, K.M.; Malaika, A.; Pereira, M.F.R.; Figueiredo, J.L. Cutting the Green Waste. Structure-Performance Relationship in Functionalized Carbon Xerogels for Hydrolysis of Cellobiose. ChemCatChem 2018, 10, 4934–4946. [Google Scholar] [CrossRef]
- Kong-Win Chang, J.; Duret, X.; Berberi, V.; Zahedi-Niaki, H.; Lavoie, J.-M. Two-Step Thermochemical Cellulose Hydrolysis with Partial Neutralization for Glucose Production. Front. Chem. 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegner, J.; Pereira, K.C.; DeBoef, B.; Lucht, B.L. Conversion of cellulose to glucose and levulinic acid via solid-supported acid catalysis. Tetrahedron Lett. 2010, 51, 2356–2358. [Google Scholar] [CrossRef]
- Chang, C.; Xu, G.; Zhu, W.; Bai, J.; Fang, S. One-pot production of a liquid biofuel candidate—Ethyl levulinate from glucose and furfural residues using a combination of extremely low sulfuric acid and zeolite USY. Fuel 2015, 140, 365–370. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Pereira, M.F.R.; Figueiredo, J.L. One-pot oxidation of cellobiose to gluconic acid. Unprecedented high selectivity on bifunctional gold catalysts over mesoporous carbon by integrated texture and surface chemistry optimization. Appl. Catal. B-Environ. 2016, 184, 381–396. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rass, H.A.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2-Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Yu, Y.; Wu, H. Tuning glucose decomposition in hot-compressed gamma-valerolactone/water mixtures: From isomerization to dehydration reactions. Fuel 2019, 238, 225–231. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Huang, K.; Fang, Z. Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3. Fuel 2013, 113, 625–631. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Venugopal, A.K.; Lucas, N.; Manikandan, M.; Thirumalaiswamy, R.; Chilukuri, S. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 2015, 5, 1463–1472. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Bior. 2019, 13, 153–173. [Google Scholar] [CrossRef] [Green Version]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.-K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium oxides: Correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Shahangi, F.; Najafi Chermahini, A.; Saraji, M. Dehydration of fructose and glucose to 5-hydroxymethylfurfural over Al-KCC-1 silica. J. Energy Chem. 2018, 27, 769–780. [Google Scholar] [CrossRef] [Green Version]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [Green Version]
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Kim, M.; Su, Y.; Fukuoka, A.; Hensen, E.J.M.; Nakajima, K. Aerobic Oxidation of 5-(Hydroxymethyl)furfural Cyclic Acetal Enables Selective Furan-2,5-dicarboxylic Acid Formation with CeO2-Supported Gold Catalyst. Angew. Chem. Int. Ed. 2018, 57, 8235–8239. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Dallas Swift, T.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef] [Green Version]
- Swift, T.D.; Bagia, C.; Choudhary, V.; Peklaris, G.; Nikolakis, V.; Vlachos, D.G. Kinetics of Homogeneous Brønsted Acid Catalyzed Fructose Dehydration and 5-Hydroxymethyl Furfural Rehydration: A Combined Experimental and Computational Study. ACS Catal. 2014, 4, 259–267. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and Brønsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Leitner, W. An overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: A rational selection of solvents using COSMO-RS and selection guides. Green Chem. 2020. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, T.; Shanks, B.H.; Datye, A.K. Tuning the Location of Niobia/Carbon Composites in a Biphasic Reaction: Dehydration of d-Glucose to 5-Hydroxymethylfurfural. Catal. Lett. 2013, 143, 509–516. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Murayama, T.; Chen, J.; Hirata, J.; Matsumoto, K.; Ueda, W. Hydrothermal synthesis of octahedra-based layered niobium oxide and its catalytic activity as a solid acid. Catal. Sci. Technol. 2014, 4, 4250–4257. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Luisa Marin, M.; Hallett-Tapley, G.L.; Impellizzeri, S.; Fasciani, C.; Simoncelli, S.; Netto-Ferreira, J.C.; Scaiano, J.C. Synthesis, acid properties and catalysis by niobium oxide nanostructured materials. Catal. Sci. Technol. 2014, 4, 3044–3052. [Google Scholar] [CrossRef] [Green Version]
- Santos, K.M.A.; Albuquerque, E.M.; Innocenti, G.; Borges, L.E.P.; Sievers, C.; Fraga, M.A. The Role of Brønsted and Water-Tolerant Lewis Acid Sites in the Cascade Aqueous-Phase Reaction of Triose to Lactic Acid. ChemCatChem 2019, 11, 3054–3063. [Google Scholar] [CrossRef]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.N.; Kondo, J.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Lebarbier, V.; Houalla, M.; Onfroy, T. New insights into the development of Brønsted acidity of niobic acid. Catal. Today 2012, 192, 123–129. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Rashed, M.N.; Ali, M.A.; Toyao, T.; Hirunsit, P.; Ehara, M.; Shimizu, K.-I. Lewis Acid Catalysis of Nb2O5 for Reactions of Carboxylic Acid Derivatives in the Presence of Basic Inhibitors. ChemCatChem 2019, 11, 383–396. [Google Scholar] [CrossRef]
- De Carvalho, G.S.G.; Chagas, L.H.; Fonseca, C.G.; de Castro, P.P.; Sant’Ana, A.C.; Leitão, A.A.; Amarante, G.W. Nb2O5 supported on mixed oxides catalyzed oxidative and photochemical conversion of anilines to azoxybenzenes. New J. Chem. 2019, 43, 5863–5871. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Xia, Q.; Liu, X.; Wang, Y. Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites. Chem. Eng. J. 2018, 332, 528–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct conversion of glucose to 5-hydroxymethylfurfural using a mixture of niobic acid and niobium phosphate as a solid acid catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.-Z.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Catalytic dehydration of fructose to 5-hydroxymethylfurfural over Nb2O5 catalyst in organic solvent. Carbohydr. Res. 2013, 368, 78–83. [Google Scholar] [CrossRef]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Wang, Y.; Wang, X.; Qu, H.; Zhao, J.; Li, Y.; Gavrilyuk, A. Hydrogen photochromism in Nb2O5 powders. Phys. Chem. Chem. Phys. 2014, 16, 20828–20833. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.; Cartraud, P.; Magnoux, P.; Guisnet, M. Textural, acidic and catalytic properties of niobium phosphate and of niobium oxide: Influence of the pretreatment temperature. Appl. Catal. A-Gen. 1992, 89, 143–153. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Wu, C.; Liang, H.; Maier, J.; Yu, Y.; Li, L. Highly Reversible and Durable Na Storage in Niobium Pentoxide through Optimizing Structure, Composition, and Nanoarchitecture. Adv. Mater. 2017, 29, 1605607–1605613. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Popović, S.; Musić, S. Sol–gel synthesis and characterization of Nb2O5 powders. Mater. Lett. 2004, 58, 2658–2663. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 catalysts. Nano Rev. 2012, 3, 17631–17642. [Google Scholar] [CrossRef] [Green Version]
- Tokio, I.; Kazuharu, O.; Kozo, T. Acidic and Catalytic Properties of Niobium Pentaoxide. Bull. Chem. Soc. Jpn. 1983, 56, 2927–2931. [Google Scholar]
- Zhou, Y.; Qiu, Z.; Lü, M.; Zhang, A.; Ma, Q. Preparation and characterization of porous Nb2O5 nanoparticles. Mater. Res. Bull. 2008, 43, 1363–1368. [Google Scholar] [CrossRef]
- Chan, X.; Pu, T.; Chen, X.; James, A.; Lee, J.; Parise, J.B.; Kim, D.H.; Kim, T. Effect of niobium oxide phase on the furfuryl alcohol dehydration. Catal. Commun. 2017, 97, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Pilarek, B.; Pelczarska, A.J.; Szczygieł, I. Characterization of niobium(v) oxide received from different sources. J. Therm. Anal. Calorim. 2017, 130, 77–83. [Google Scholar] [CrossRef]
- Kodama, R.; Terada, Y.; Nakai, I.; Komaba, S.; Kumagai, N. Electrochemical and In Situ XAFS-XRD Investigation of Nb2O5 for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2006, 153, A583–A588. [Google Scholar] [CrossRef]
- Li, S.; Qian, X.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef] [Green Version]
- Silva, Â.; Wilson, K.; Lee, A.F.; dos Santos, V.C.; Cons Bacilla, A.C.; Mantovani, K.M.; Nakagaki, S. Nb2O5/SBA-15 catalyzed propanoic acid esterification. Appl. Catal. B-Environ. 2017, 205, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129–140. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Li, M.M.J.; Peng, Y.-K.; Nakagawa, K.; Hooper, T.J.N.; Hanna, J.V.; Shepherd, A.; Wu, T.-S.; Soo, Y.-L.; Tsang, S.C.E. Structural Studies of Bulk to Nanosize Niobium Oxides with Correlation to Their Acidity. J. Am. Chem. Soc. 2017, 139, 12670–12680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Aghamohammadi, S.; Li, D.; Li, K.; Farrauto, R. Structure dependence of Nb2O5-X supported manganese oxide for catalytic oxidation of propane: Enhanced oxidation activity for MnOx on a low surface area Nb2O5-X. Appl. Catal. B-Environ. 2019, 244, 438–447. [Google Scholar] [CrossRef]
- Teles, C.A.; de Souza, P.M.; Rabelo-Neto, R.C.; Griffin, M.B.; Mukarakate, C.; Orton, K.A.; Resasco, D.E.; Noronha, F.B. Catalytic upgrading of biomass pyrolysis vapors and model compounds using niobia supported Pd catalyst. Appl. Catal. B-Environ. 2018, 238, 38–50. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Kumar, C.P.; Murali, A.; Tripathi, A.; Clearfield, A. Studies on catalytic functionality of V2O5/Nb2O5 catalysts. J. Mol. Catal. A Chem. 2004, 216, 139–146. [Google Scholar] [CrossRef]
- Candu, N.; El Fergani, M.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Li, K.; Jia, X.; Wang, B.; Borgna, A.; Yang, Y. Titania-Supported Gold Nanoparticles as Efficient Catalysts for the Oxidation of Cellobiose to Organic Acids in Aqueous Medium. ChemCatChem 2014, 6, 2105–2114. [Google Scholar] [CrossRef]
- Wang, S.-X.; Guo, R.-T.; Pan, W.-G.; Chen, Q.-L.; Sun, P.; Li, M.-Y.; Liu, S.-M. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals: TPD and DRIFT studies. Catal. Commun. 2017, 89, 143–147. [Google Scholar] [CrossRef]
- Aegerter, M.A. Sol–gel niobium pentoxide: A promising material for electrochromic coatings, batteries, nanocrystalline solar cells and catalysis. Sol. Energy Mater. Sol. Cells 2001, 68, 401–422. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, S.; Kurosaki, A. Acidic properties and catalytic activities of niobic acid treated with phosphoric acid. Catal. Today 1990, 8, 113–122. [Google Scholar] [CrossRef]
- Ushikubo, T. Recent topics of research and development of catalysis by niobium and tantalum oxides. Catal. Today 2000, 57, 331–338. [Google Scholar] [CrossRef]
- Portillo Perez, G.; Mukherjee, A.; Dumont, M.-J. Insights into HMF catalysis. J. Ind. Eng.Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ren, J.; Liu, X.; Li, X.; Xia, Y.; Lu, G.; Wang, Y. Mesoporous niobium phosphate: An excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Wang, B.; Li, L.; Zhong, H. One-pot synthesis of 5-hydroxymethylfurfural from glucose over zirconium doped mesoporous KIT-6. Chin. J. Chem. Eng. 2018, 26, 1270–1277. [Google Scholar] [CrossRef]
- Guo, B.; Ye, L.; Tang, G.; Zhang, L.; Yue, B.; Tsang, S.C.E.; He, H. Effect of Brønsted/Lewis Acid Ratio on Conversion of Sugars to 5-Hydroxymethylfurfural over Mesoporous Nb and Nb-W Oxides. Chin. J. Chem. 2017, 35, 1529–1539. [Google Scholar] [CrossRef]
- Noma, R.; Nakajima, K.; Kamata, K.; Kitano, M.; Hayashi, S.; Hara, M. Formation of 5-(Hydroxymethyl)furfural by Stepwise Dehydration over TiO2 with Water-Tolerant Lewis Acid Sites. J. Phys. Chem. C 2015, 119, 17117–17125. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Acid Catalysts in a Biphasic System. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Ordomsky, V.V.; Sushkevich, V.L.; Schouten, J.C.; van der Schaaf, J.; Nijhuis, T.A. Glucose dehydration to 5-hydroxymethylfurfural over phosphate catalysts. J. Catal. 2013, 300, 37–46. [Google Scholar] [CrossRef]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef] [Green Version]












| Sample | SBET (m2/g) | Smeso (m2/g) | Vtot (cm3/g) |
|---|---|---|---|
| Nb2O5·nH2O | 366 | 366 | 0.27 |
| Nb2O5_300_air | 209 | 209 | 0.23 |
| Nb2O5_550_air | 27 | 27 | 0.20 |
| Nb2O5_550_N2 | 39 | 39 | 0.13 |
| Nb2O5_SA | 4 | 4 | 0.12 |
| Sample | Nb3d (At%) | O1s (At%) | O/Nb | Atot (mmol/g) |
|---|---|---|---|---|
| Nb2O5·nH2O | 25.76 | 74.24 | 2.88 | 2.35 |
| Nb2O5_300_air | 25.97 | 74.03 | 2.85 | 1.69 |
| Nb2O5_550_air | 26.61 | 73.39 | 2.76 | 0.19 |
| Nb2O5_550_N2 | 26.69 | 73.31 | 2.75 | 0.05 |
| Nb2O5_SA | 26.81 | 73.19 | 2.73 | 0.02 |
| Sample | O(I) (%) | O(II) (%) | O(III) (%) | O(II)/O(I) |
|---|---|---|---|---|
| Nb2O5·nH2O | 83.96 | 10.08 | 5.96 | 0.12 |
| Nb2O5_300_air | 83.26 | 11.33 | 5.41 | 0.14 |
| Nb2O5_550_air | 86.84 | 8.18 | 4.98 | 0.09 |
| Nb2O5_550_N2 | 85.81 | 8.96 | 5.23 | 0.10 |
| Nb2O5_SA | 85.26 | 9.80 | 4.94 | 0.11 |
| Sample | Tmax (°C) | Weak (%) | Tmax (°C) | Medium (%) | Tmax (°C) | Strong (%) |
|---|---|---|---|---|---|---|
| Nb2O5_SA | 0 | 325 | 53 | 410 | 47 | |
| Nb2O5_300_air | 270 | 22 | 315 | 33 | 394 | 45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawa Eblagon, K.; Malaika, A.; Ptaszynska, K.; Pereira, M.F.R.; Figueiredo, J.L. Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials 2020, 10, 1685. https://doi.org/10.3390/nano10091685
Morawa Eblagon K, Malaika A, Ptaszynska K, Pereira MFR, Figueiredo JL. Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials. 2020; 10(9):1685. https://doi.org/10.3390/nano10091685
Chicago/Turabian StyleMorawa Eblagon, Katarzyna, Anna Malaika, Karolina Ptaszynska, Manuel Fernando R. Pereira, and José Luís Figueiredo. 2020. "Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water" Nanomaterials 10, no. 9: 1685. https://doi.org/10.3390/nano10091685
APA StyleMorawa Eblagon, K., Malaika, A., Ptaszynska, K., Pereira, M. F. R., & Figueiredo, J. L. (2020). Impact of Thermal Treatment of Nb2O5 on Its Performance in Glucose Dehydration to 5-Hydroxymethylfurfural in Water. Nanomaterials, 10(9), 1685. https://doi.org/10.3390/nano10091685