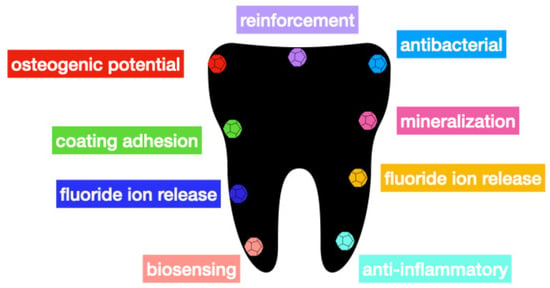
Nanomaterials in Dentistry: State of the Art and Future Challenges
Abstract
:1. Introduction
2. Zeolites
3. Graphene
4. Nanorods, Nanowires, and Nanotubes
5. Conclusions, Perspectives and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, R.; Van Baten, J. In silico screening of metal–organic frameworks in separation applications. Phys. Chem. Chem. Phys. 2011, 13, 10593–10616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Nalwa, H.S. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.; Kim, J.-H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Nalwa, H.S. A Special Issue on Reviews in Biomedical Applications of Nanomaterials, Tissue Engineering, Stem Cells, Bioimaging, and Toxicity. J. Biomed. Nanotechnol. 2014, 10, 2421–2423. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.Q.; Zou, Z.F.; Si, C.L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Zheng, X.; Pan, Y.; Wang, Z.; Zhang, M.; Chen, Q.; Dong, W.; Chen, L. Janus “nano-bullets” for magnetic targeting liver cancer chemotherapy. Biomaterials 2016, 100, 118–133. [Google Scholar] [CrossRef]
- Goldberg, M.; Langer, R.; Jia, Z. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [Green Version]
- Suh, W.H.; Suslick, K.S.; Stucky, G.D.; Suh, Y.-H. Nanotechnology, nanotoxicology, and neuroscience. Prog. Neurobiol. 2009, 87, 133–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopp, D.; Tang, D.; Niessner, R. Review: Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 2009, 647, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, Crystallization Mechanism, and Applications. Chem. Mater. 2005, 17, 2494–2513. [Google Scholar] [CrossRef]
- Li, Q.; Creaser, D.; Sterte, J. An Investigation of the Nucleation/Crystallization Kinetics of Nanosized Colloidal Faujasite Zeolites. Chem. Mater. 2002, 14, 1319–1324. [Google Scholar] [CrossRef]
- Holmberg, B.A.; Wang, H.; Yan, Y. High silica zeolite Y nanocrystals by dealumination and direct synthesis. Microporous Mesoporous Mater. 2004, 74, 189–198. [Google Scholar] [CrossRef]
- Rad, L.R.; Momeni, A.; Ghazani, B.F.; Irani, M.; Mahmoudi, M.; Noghreh, B. Removal of Ni2+ and Cd2+ ions from aqueous solutions using electrospun PVA/zeolite nanofibrous adsorbent. Chem. Eng. J. 2014, 256, 119–127. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Nemcakova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef]
- Okulus, Z.; Sandomierski, M.; Zielinska, M.; Buchwald, Z.; Voelkel, A. Zeolite fillers for resin-based composites with remineralizing potential. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Kadir, M.R.A.; Iqbal, S.; Razak, S.I.A.; Rafique, M.S.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Khattak, M.A.; Raghavendran, H.R.B.; Abbas, A.A. Nano-hydroxyapatite reinforced zeolite ZSM composites: A comprehensive study on the structural and in vitro biological properties. Ceram. Int. 2016, 42, 7175–7182. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, J.S.; Kim, K.H.; Kwon, T.Y. Antimicrobial Activity of Glass Ionomer Cement Incorporated with Chlorhexidine-Loaded Zeolite Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Pal, S.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [Green Version]
- Ure, D.; Harris, J. Nanotechnology in dentistry: Reduction to practice. Dent. Updat. 2003, 30, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tirupathi, S.; Svsg, N.; Rajasekhar, S.; Nuvvula, S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 2019, 11, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Yamamoto, T.; Hamada, T.; Rahardjo, M.; Murata, H.; Nakanoda, S. Antifungal effect of zeolite-incorporated tissue conditioner against Candida albicans growth and/or acid production. J. Oral Rehabil. 2008, 24, 350–357. [Google Scholar] [CrossRef]
- Cinar, C.; Odabas, M.; Gurel, M.A.; Baldag, I. The effects of incorporation of silver-zeolite on selected properties of mineral trioxide aggregate. Dent. Mater. J. 2013, 32, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Odabas, M.E.; Çınar, C.; Akca, G.; Araz, I.; Ulusu, T.; Yucel, H. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent. Traumatol. 2011, 27, 189–194. [Google Scholar] [CrossRef]
- Cinar, C.; Ulusu, T.; Ozcelik, B.; Karamuftuoglu, N.; Yucel, H. Antibacterial effect of silver-zeolite containing root-canal filling material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 592–595. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Ueshige, M.; Abe, Y.; Sato, Y.; Tsuga, K.; Akagawa, Y.; Ishii, M. Dynamic viscoelastic properties of antimicrobial tissue conditioners containing silver-zeolite. J. Dent. 1999, 27, 517–522. [Google Scholar] [CrossRef]
- Abe, Y.; Ishii, M.; Takeuchi, M.; Ueshige, M.; Tanaka, S.; Akagawa, Y. Effect of saliva on an antimicrobial tissue conditioner containing silver-zeolite. J. Oral Rehabil. 2004, 31, 568–573. [Google Scholar] [CrossRef]
- Ghatole, K.; Patil, A.; Giriyappa, R.H.; Singh, T.V.; Jyotsna, S.V.; Rairam, S. Evaluation of Antibacterial Efficacy of MTA with and without Additives Like Silver Zeolite and Chlorhexidine. J. Clin. Diagn. Res. 2016, 10, 11–14. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samiei, M.; Ghasemi, N.; Asl-Aminabadi, N.; Divband, B.; Golpavar-Dashti, Y.; Shirazi, S. Nanoparticles and Zeolites: Antibacterial Effects and their Mechanism against Pathogens. J. Clin. Exp. Dent. 2017, 9, 356–360. [Google Scholar] [CrossRef]
- Kusy, R.P.; Whitley, J.Q.; Kalachandra, S. Mechanical properties and interrelationships of poly(methyl methacrylate) following hydration over saturated salts. Polymer 2001, 42, 2585–2595. [Google Scholar] [CrossRef]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3, 5771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malic, S.; Rai, S.; Redfern, J.; Pritchett, J.; Liauw, C.M.; Verran, J.; Tosheva, L. Zeolite-embedded silver extends antimicrobial activity of dental acrylics. Colloids Surf. B Biointerfaces 2019, 173, 52–57. [Google Scholar] [CrossRef]
- Saravanan, M.; Kumar, V.; Padmanabhan, T.V.; Banu, F. Viscoelastic Properties and Antimicrobial Effects of Soft Liners with Silver Zeolite in Complete Dental Prosthesis Wearers: An In Vivo Study. Int. J. Prosthodont. 2016, 28, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Casemiro, L.A.; Martins, C.H.G.; Pires-De-Souza, F.D.C.P.; Panzeri, H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc zeolite—Part I. Gerodontology 2008, 25, 187–194. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Pei, X.; Wang, J.; Wan, Q.; Jiang, S.; Huang, C.; Pei, X. Enhanced Osseointegration of Porous Titanium Modified with Zeolitic Imidazolate Framework-8. ACS Appl. Mater. Interfaces 2017, 9, 25171–25183. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huang, C.; Cai, H.; Hu, S.; Wan, Q.; Pei, X.; Wang, J. Osteogenic activity and antibacterial effect of porous titanium modified with metal-organic framework films. Biomed. J. Mater. Res. A 2016, 105, 834–846. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 19, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantu, Y.I.; Rodriguez-Macias, F.; Elías, A.L.; Muñoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.C. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.-V.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hagh, H.B.K.; Azhar, F.F. Reinforcing materials for polymeric tissue engineering scaffolds: A review. Biomed. J. Mater. Res. B Appl. Biomater. 2018, 107, 1560–1575. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, D.; Ma, Z.; Cao, W.; Hou, Y.; Zhu, J.; Gan, Y.; Yang, M. An Epidermis-like Hierarchical Smart Coating with a Hardness of Tooth Enamel. ACS Nano 2018, 12, 1062–1073. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Rodríguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, 19343. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Bae, H.; Chu, H.; Edalat, F.; Cha, J.M.; Sant, S.; Kashyap, A.; Ahari, A.F.; Kwon, C.H.; Nichol, J.W.; Manoucheri, S.; et al. Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2012, 8, 1–14. [Google Scholar] [CrossRef]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface Modification of Multipass Caliber-Rolled Ti Alloy with Dexamethasone-Loaded Graphene for Dental Applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Upadhyay, J.; Das, R. Fillers and Reinforcements for Advanced Nanocomposites, 1st ed.; Wookhead Publishing Series in Composites Science and Engineering Chapter 8; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2015; pp. 157–232. ISBN 9780081000793. [Google Scholar]
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial Activity against Staphylococcus Aureus of Titanium Surfaces Coated with Graphene Nanoplatelets to Prevent Peri-Implant Diseases. An In-Vitro Pilot Study. Int. J. Environ. Res. Public Heal. 2020, 17, 1568. [Google Scholar] [CrossRef] [Green Version]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Malhotra, R.; Han, Y.M.; Morin, J.L.P.; Luong-Van, E.K.; Chew, R.J.J.; Neto, A.C.; Nijhuis, C.; Rosa, V. Inhibiting Corrosion of Biomedical-Grade Ti-6Al-4V Alloys with Graphene Nanocoating. J. Dent. Res. 2020, 99, 285–292. [Google Scholar] [CrossRef]
- Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M.; Nishida, E. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar] [CrossRef] [Green Version]
- Mohammadrezaei, D.; Golzar, H.; Rad, M.R.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Lv, L.; Du, F.; Niu, T.; Chen, T.; Xia, D.; Wang, S.; Zhao, X.; Liu, J.; Xiong, C.; et al. Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 2018, 25, 8141. [Google Scholar] [CrossRef]
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 107, 635–645. [Google Scholar] [CrossRef]
- Zeng, Y.; Pei, X.; Yang, S.; Qin, H.; Cai, H.; Hu, S.; Sui, L.; Wan, Q.; Wang, J. Graphene oxide/hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Liu, M.; Hao, L.; Huang, Q.; Zhao, D.; Li, Q.; Cai, X. Tea Polyphenol-Reduced Graphene Oxide Deposition on Titanium Surface Enhances Osteoblast Bioactivity. J. Nanosci. Nanotechnol. 2018, 18, 3134–3140. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Meena, R.; Singh, B.R.; Khan, A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 2014, 30, 1281–1294. [Google Scholar] [CrossRef]
- Lee, S.M.; Yoo, K.H.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Son, W.S.; Ko, C.C.; Son, S.A.; Kim, Y.I. Enamel Anti-Demineralization Effect of Orthodontic Adhesive Containing Bioactive Glass and Graphene Oxide: An In-Vitro Study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [Green Version]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense vicR RNA reduced exopolysaccharide synthesis and biofilm aggregation for Streptococcus mutans. Dent. Mater. J. 2020, 39, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The Antibacterial Effect of Graphene Oxide on Streptococcus mutans. J. Nanosci. Nanotechnol. 2020, 20, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Y.; Xie, Y.; Yu, C.H.; Chen, G.Y.; Li, Y.H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control Release 2019, 307, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhao, Q.; Lu, S.; Fu, Y.; Yu, N.; Zhao, W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2018, 27, e20180042. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.M.; Lin, J.C.; Chen, Z.Y.; Wei, M.C.; Fu, Y.X.; Lu, S.S.; Yu, D.-S.; Zhao, W. Enhanced antimicrobial activities of silver-nanoparticle-decorated reduced graphene nanocomposites against oral pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef]
- La, W.G.; Park, S.; Yoon, H.H.; Jeong, G.J.; Lee, T.J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.S. Delivery of a Therapeutic Protein for Bone Regeneration from a Substrate Coated with Graphene Oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef]
- Vera-Sánchez, M.; Aznar-Cervantes, S.; Jover, E.; García-Bernal, D.; Oñate-Sánchez, R.E.; Hernández-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, I.R.; Kim, Y.; Kim, K.H.; Park, S.; Park, B.-S.; Bae, M.-K.; Kim, Y.I. The Effect of Mesoporous Bioactive Glass Nanoparticles/Graphene Oxide Composites on the Differentiation and Mineralization of Human Dental Pulp Stem Cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; García-Bernal, D.; Aznar-Cervantes, S.D.; Ros-Roca, M.A.; Algueró, M.C.; Atucha, N.M.; Lozano-García, A.A.; Moraleda, J.M.; Cenis, J.L. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. J. Mater. Sci. Mater. Electron. 2014, 25, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, R.; Zara, S.; Ventrella, A.; Siani, G.; Da Ros, T.; Iezzi, G.; Cataldi, A.; Fontana, A. Covalent Decoration of Cortical Membranes with Graphene Oxide as a Substrate for Dental Pulp Stem Cells. Nanomaterials 2019, 9, 604. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Neto, A.H.C.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017, 33, 13–21. [Google Scholar] [CrossRef]
- Cucchi, A.; Ghensi, P. Vertical Guided Bone Regeneration using Titanium-reinforced d-PTFE Membrane and Prehydrated Corticocancellous Bone Graft. Open Dent. J. 2014, 8, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Malik, S.; Ruddock, F.M.; Dowling, A.H.; Byrne, K.; Schmitt, W.; Khalakhan, I.; Nemoto, Y.; Guo, H.; Shrestha, L.K.; Ariga, K.; et al. Graphene composites with dental and biomedical applicability. Beilstein J. Nanotechnol. 2018, 9, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef] [Green Version]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [Green Version]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Graphene Oxide/Silver Nanoparticle Coating Produced by Electrophoretic Deposition Improved the Mechanical and Tribological Properties of NiTi Alloy for Biomedical Applications. J. Nanosci. Nanotechnol. 2019, 19, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; Del Real, J.; Dunne, N.J. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Morales-Narváez, E.; Merkoçi, A. Graphene Oxide as an Optical Biosensing Platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Ren, S.; Fan, R.; Yu, Y.; Zhang, H.; Liu, C.; Miao, L. Functionalized titanium implant in regulating bacteria and cell response. Int. J. Nanomed. 2019, 14, 1433–1450. [Google Scholar] [CrossRef] [Green Version]
- Son, S.A.; Kim, D.H.; Yoo, K.H.; Yoon, S.Y.; Kim, Y.I. Mesoporous Bioactive Glass Combined with Graphene Oxide Quantum Dot as a New Material for a New Treatment Option for Dentin Hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef] [Green Version]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. V-Containing Mixed Oxide Catalysts for Reduction–Oxidation-Based Reactions with Environmental Applications: A Short Review. Catalysts 2018, 8, 564. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Tian, J.; Wang, L.; Li, H.; Zhang, Y.; Sun, X. Stable Aqueous Dispersion of Graphene Nanosheets: Noncovalent Functionalization by a Polymeric Reducing Agent and Their Subsequent Decoration with Ag Nanoparticles for Enzymeless Hydrogen Peroxide Detection. Macromolecules 2010, 43, 10078–10083. [Google Scholar] [CrossRef]
- Su, I.-H.; Lee, C.-F.; Su, Y.-P.; Wang, L.-H.; Paravina, R.D. Evaluating a Cobalt-Tetraphenylporphyrin Complex, Functionalized with a Reduced Graphene Oxide Nanocomposite, for Improved Tooth Whitening. J. Esthet. Restor. Dent. 2016, 28, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Berenguer, R.; Guerrero-Pérez, M.O.; Guzmán, I.; Rodríguez-Mirasol, J.; Cordero, T. Synthesis of Vanadium Oxide Nanofibers with Variable Crystallinity and V5+/V4+ Ratios. ACS Omega 2017, 2, 7739–7745. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Seeram, R.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- De Vasconcellos, L.M.R.; Prado, R.F.D.; Sartori, E.; Mendonça, D.B.S.; Mendonça, G.; Marciano, F.R.; Lobo, A.O. In vitro osteogenesis process induced by hybrid nanohydroxyapatite/graphene nanoribbons composites. J. Mater. Sci. Mater. Med. 2019, 30, 81. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Development of a multiwalled carbon nanotube coated collagen dish. Dent. Mater. J. 2009, 28, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nahorny, S.; Zanin, H.; Christino, V.A.; Marciano, F.R.; Lobo, A.O.; Soares, L.E.S. Multi-walled carbon nanotubes/graphene oxide hybrid and nanohydroxyapatite composite: A novel coating to prevent dentin erosion. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 199–208. [Google Scholar] [CrossRef]
- Meng, Y.H.; Tang, C.-Y.; Tsui, C.P.; Chen, D.Z. Fabrication and characterization of needle-like nano-HA and HA/MWNT composites. J. Mater. Sci. Mater. Med. 2007, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Simonovic, J.; Tolijic, B.; Nikolic, N.; Peric, M.; Vujin, J.; Panajotovic, R.; Gajic, R.; Bekyarova, E.; Cataldi, A.; Parpura, V.; et al. Differentiation of stem cells from apical papilla into neural lineage using graphene dispersion and single walled carbon nanotubes. J. Biomed. Mater. Res. A 2018, 106, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Watari, F.; Omori, M.; Liao, S.; Zhu, Y.; Yokoyama, A.; Uo, M.; Kimura, H.; Ohkubo, A. Mechanical properties and biological behavior of carbon nanotube/polycarbosilane composites for implant materials. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.K.; Low, K.L.; Zein, S.H.S.; McPhail, D.S.; Gerhardt, L.C.; Roether, J.A.; Boccaccini, A.R. Reinforcement of calcium phosphate cement with multi-walled carbon nanotubes and bovine serum albumin for injectable bone substitute applications. J. Mech. Behav. Biomed. Mater. 2011, 4, 331–339. [Google Scholar] [CrossRef]
- Maho, A.; Linden, S.; Arnould, C.; Detriche, S.; Delhalle, J.; Mekhalif, Z. Tantalum oxide/carbon nanotubes composite coatings on titanium, and their functionalization with organophosphonic molecular films: A high quality scaffold for hydroxyapatite growth. J. Colloid Interface Sci. 2012, 371, 150–158. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Xu, S. Progress in Research on Carbon Nanotubes Reinforced Cementitious Composites. Adv. Mater. Sci. Eng. 2015, 307435. [Google Scholar] [CrossRef] [Green Version]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Marrs, B.; Andrews, R.; Rantell, T.; Pienkowski, D. Augmentation of acrylic bone cement with multiwall carbon nanotubes. J. Biomed. Mater. Res. A 2006, 77, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, S.A.; Palasuk, J.; Kamocki, K.; Geraldeli, S.; Gregory, R.L.; Platt, J.A.; Windsor, L.J.; Bottino, M.C. Doxycycline-Encapsulated Nanotube-Modified Dentin Adhesives. J. Dent. Res. 2014, 93, 1270–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barot, T.; Rawtani, D.; Kulkarni, P.; Hussain, C.M.; Akkireddy, S. Physicochemical and biological assessment of flowable resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103675. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, A.; Vardaki, M.; Mihai, G.; Ionita, D.; Stoian, A.B.; Enachescu, M.; Demetrescu, I. Understanding surface and interface properties of modified Ti50Zr with nanotubes. Appl. Surf. Sci. 2020, 506, 14461. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Xu, L.; Gu, N. Surface modification and microstructure of single-walled carbon nanotubes for dental resin-based composites. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 90–97. [Google Scholar] [CrossRef]
- Garmendia, N.; Grandjean, S.; Chevalier, J.; Diaz, L.; Torrecillas, R.; Obieta, I. Zirconia–multiwall carbon nanotubes dense nano-composites with an unusual balance between crack and ageing resistance. J. Eur. Ceram. Soc. 2011, 31, 1009–1014. [Google Scholar] [CrossRef]
- Marković, Z.; Harhaji-Trajkovic, L.; Marković, B.T.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Silva-Tapia, A.B.; García, X.; Radovic, L.R. Similarities and differences in O2 chemisorption on graphene nanoribbon vs. carbon nanotube. Carbon 2012, 50, 1152–1162. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, S.; Lee, Y.S. Effect of the addition of carbon black and carbon nanotube to FeS2 cathode on the electrochemical performance of thermal battery. J. Ind. Eng. Chem. 2014, 20, 3584–3589. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jin, S. Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 2006, 26, 1301–1306. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania Nanotubes: A Novel Platform for Drug-Eluting Coatings for Medical Implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2016, 14, 1009–1024. [Google Scholar] [CrossRef]
- Balasundaram, G.; Yao, C.; Webster, T.J. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J. Biomed. Mater. Res. A 2007, 84, 447–453. [Google Scholar] [CrossRef]
- Kodama, A.; Bauer, S.; Komatsu, A.; Asoh, H.; Ono, S.; Schmuki, P. Bioactivation of titanium surfaces using coatings of TiO2 nanotubes rapidly pre-loaded with synthetic hydroxyapatite. Acta Biomater. 2009, 5, 2322–2330. [Google Scholar] [CrossRef]
- Cao, X.; Yu, W.-Q.; Qiu, J.; Zhao, Y.-F.; Zhang, Y.-L.; Zhang, F.-Q. RGD peptide immobilized on TiO2 nanotubes for increased bone marrow stromal cells adhesion and osteogenic gene expression. J. Mater. Sci. Mater. Med. 2011, 23, 527–536. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Sinha-Ray, S.; Sukotjo, C.; Yarin, A.L. Intercalation of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2013, 3, 17380–17836. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Chen, S.; Lu, R.; Li, H.; Zhang, Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomed. 2017, 12, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Su, P.; Chen, S.; Wang, N.; Ma, Y.; Liu, Y.; Wang, J.; Zhang, Z.; Li, H.; Webster, T.J. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-S.; Park, Y.-B.; Bae, J.-M.; Oh, S. Near-infrared laser-mediated drug release and antibacterial activity of gold nanorod-sputtered titania nanotubes. J. Tissue Eng. 2018, 9, 2041731418790315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Gao, A.; Huang, X.; Wang, X.; Zhang, X.-Y.; Qin, L.; Tang, B. Antibacterial activity and cytocompatibility of Cu-Ti-O nanotubes. J. Biomed. Mater. Res. A 2013, 102, 1850–1858. [Google Scholar] [CrossRef]
- Khaled, S.M.Z.; Miron, R.J.; Hamilton, D.W.; Charpentier, P.A.; Rizkalla, A.S. Reinforcement of resin based cement with titania nanotubes. Dent. Mater. 2010, 26, 169–178. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Lucena, F.S.; Borges, A.F.S.; Lisboa-Filho, P.N.; Furuse, A.Y. Incorporation of TiO2 nanotubes in a polycrystalline zirconia: Synthesis of nanotubes, surface characterization, and bond strength. J. Prosthet. Dent. 2018, 120, 589–595. [Google Scholar] [CrossRef]
- Berenguer, R.; Fornells, J.; García-Mateos, F.J.; Guerrero-Pérez, M.; Rodríguez-Mirasol, J.; Cordero, T. Novel Synthesis Method of porous VPO catalysts with fibrous structure by Electrospinning. Catal. Today 2016, 277, 266–273. [Google Scholar] [CrossRef]
- Hidalgo, J.J.T.; Liñán, J.T.; Guerrero-Pérez, M.O.; Rodríguez-Mirasol, J.; Cordero, T. Electrospun vanadium oxide based submicron diameter fiber catalysts. Part I: Preparation procedure and propane ODH application. Catal. Today 2019, 325, 131–143. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Sharifi, S.; Jahangiri, A. Electrospun nanofibers as versatile platform in antimicrobial delivery: Current state and perspectives. Pharm. Dev. Technol. 2019, 24, 1187–1199. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging Biomedical Applications of Nano-Chitins and Nano-Chitosans Obtained via Advanced Eco-Friendly Technologies from Marine Resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Hench, L.L.; Du, J.; Choy, K.L. Preparation of Hydroxyapatite Fibers by Electrospinning Technique. J. Am. Ceram. Soc. 2005, 87, 1988–1991. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, C.K.S.; Sharma, C.P. Production, Properties and Applications of Fibres from Chitin and Chitosan. Trends Biomater. Artif. Organs 2018, 32, 23–82. Available online: https://www.biomaterials.org.in/tibao/index.php/tibao/article/view/318 (accessed on 7 September 2020).
- Mao, L.X.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015, 10, 7031–7044. [Google Scholar] [CrossRef] [Green Version]
- Kehr, N.S.; Atay, S.; Ergün, B. Self-assembled Monolayers and Nanocomposite Hydrogels of Functional Nanomaterials for Tissue Engineering Applications. Macromol. Biosci. 2014, 15, 445–463. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; Han, Y.; Zhao, L. The osteogenic capacity of biomimetic hierarchical micropore/nanorod-patterned Sr-HA coatings with different interrod spacings. Nanomedicine 2016, 12, 1161–1173. [Google Scholar] [CrossRef]
- Sartuqui, J.; Gravina, A.N.; Rial, R.; Benedini, L.A.; Yahia, L.; Ruso, J.M.; Messina, P.V. Biomimetic fiber mesh scaffolds based on gelatin and hydroxyapatite nano-rods: Designing intrinsic skills to attain bone reparation abilities. Colloids Surf. B Biointerfaces 2016, 145, 382–391. [Google Scholar] [CrossRef]
- Li, B.; Gao, P.; Zhang, H.; Guo, Z.; Zheng, Y.; Han, Y. Osteoimmunomodulation, osseointegration, and in vivo mechanical integrity of pure Mg coated with HA nanorod/pore-sealed MgO bilayer. Biomater. Sci. 2018, 6, 3202–3218. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Liu, F.; Duan, J.; Wang, S.; Han, J.; Sang, Y.; Yu, X.; Li, D.; Tang, W.; et al. One-Dimensional Hydroxyapatite Nanostructures with Tunable Length for Efficient Stem Cell Differentiation Regulation. ACS Appl. Mater. Interfaces 2017, 9, 33717–33727. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Atai, M.; Nodehi, A.; Khanlar, L.N. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Kandiah, K.; Prabhu, M.; Meenakshisundaram, N.; Rajendran, V. Nanohydroxyapatite–chitosan–gelatin polyelectrolyte complex with enhanced mechanical and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.N.; Yang, D.L.; Wang, D.; Pu, Y.; Le, Y.; Wang, J.X.; Chen, J.F. Design and efficient fabrication of micro-sized clusters of hydroxyapatite nanorods for dental resin composites. J. Mater. Sci. 2018, 54, 3878–3892. [Google Scholar] [CrossRef]
- Mishra, V.K.; Srivastava, S.K.; Asthana, B.P.; Kumar, D. Structural and spectroscopic studies of hydroxyapatite nanorods formed via microwave-assisted synthesis route. J. Am. Ceram. Soc. 2012, 95, 2709–2715. [Google Scholar] [CrossRef]
- Jevtic, M.; Mitric, M.; Skapin, S.; Jancar, B.; Ignjatovic, N.; Uskokovic, D. Crystal Structure of Hydroxyapatite Nanorods Synthesized by Sonochemical Homogeneous Precipitation. Cryst. Growth Des. 2008, 8, 2217–2222. [Google Scholar] [CrossRef]
- Kamieniak, J.; Doyle, A.M.; Kelly, P.J.; Banks, C.E. Novel synthesis of mesoporous hydroxyapatite using carbon nanorods as a hard-template. Ceram. Int. 2017, 43, 5412–5416. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Seo, Y.H.; Oh, T.H. PVP Assisted Synthesis of Hydroxyapatite Nanorods with Tunable Aspect Ratio and Bioactivity. J. Nanomater. 2015, 2015, 621785. [Google Scholar] [CrossRef]
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interface Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef]
- Chen, H.; Sun, K.; Tang, Z.; Law, R.V.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Synthesis of Fluorapatite Nanorods and Nanowires by Direct Precipitation from Solution. Cryst. Growth Des. 2006, 6, 1504–1508. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef]
- Sowmya, S.; Bumgardener, J.D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog. Polym. Sci. 2013, 38, 1748–1772. [Google Scholar] [CrossRef]
- Li, X.; Pan, D.; Lin, S.; Zhuang, Z.; Lin, Z. Facile in vitro hydroxyapatite remineralization of human enamel with remarkable hardness. CrystEngComm 2013, 15, 4351. [Google Scholar] [CrossRef]
- Neto, D.A.; Carvalho, E.V.; Rodrigues, E.; Feitosa, V.P.; Sauro, S.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Rodrigues, L.K.; Fechine, P.B.A. Novel hydroxyapatite nanorods improve anti-caries efficacy of enamel infiltrants. Dent. Mater. 2016, 32, 784–793. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Lin, K. Facile synthesis of dental enamel-like hydroxyapatite nanorod arrays via hydrothermal transformation of hillebrandite nanobelts. J. Mater. Chem. B 2015, 3, 7334–7339. [Google Scholar] [CrossRef]
- Daood, U.; Fawzy, A.S. Minimally invasive high-intensity focused ultrasound (HIFU) improves dentine remineralization with hydroxyapatite nanorods. Dent. Mater. 2020, 36, 456–467. [Google Scholar] [CrossRef]
- Tian, K.; Peng, M.; Fei, W.; Liao, C.; Ren, X. Induced Synthesis of Hydroxyapatite by Chitosan for Enamel Remineralization. Adv. Mater. Res. 2012, 530, 40–45. [Google Scholar] [CrossRef]
- Taheri, M.M.; Kadir, M.R.A.; Shokuhfar, T.; Hamlekhan, A.; Shirdar, M.R.; NaghiZadeh, F. Fluoridated hydroxyapatite nanorods as novel fillers for improving mechanical properties of dental composite: Synthesis and application. Mater. Des. 2015, 82, 119–125. [Google Scholar] [CrossRef]
- Wu, Y.R.; Chang, C.W.; Chang, K.C.; Lin, D.J.; Ko, C.L.; Wu, H.Y.; Chen, W.C. Effect of micro-/nano-hybrid hydroxyapatite rod reinforcement in composite resins on strength through thermal cycling. Polym. Compos. 2019, 40, 3703–3710. [Google Scholar] [CrossRef]
- Velo, M.M.A.C.; Nascimento, T.R.L.; Scotti, C.K.; Bombonatti, J.F.S.; Furuse, A.Y.; Silva, V.D.; Simões, T.A.; Medeiros, E.S.; Blaker, J.J.; Silikas, N.; et al. Improved mechanical performance of self-adhesive resin cement filled with hybrid nanofibers-embedded with niobium pentoxide. Dent. Mater. 2019, 35, e272–e285. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.L.; Zhu, Y.J.; Zhu, C.L.; Feng, X.P. Synthesis and antibacterial property of zinc loaded hydroxyapatite nanorods. Mater. Lett. 2012, 89, 233–235. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol–collagen–hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Q.W.; Sun, T.W.; Chen, F.; Qi, C.; Wu, J.; Cai, Z.Y.; Qian, Q.R.; Zhu, Y.J. Amorphous calcium phosphate, hydroxyapatite and poly(d,l-lactic acid) composite nanofibers: Electrospinning preparation, mineralization and in vivo bone defect repair. Colloids Surf. B Biointerfaces 2015, 136, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 107, 110347. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N. Enhanced mechanical strength of hydroxyapatite nanorods reinforced with polyethylene. J. Nanoparticle Res. 2010, 13, 1841–1853. [Google Scholar] [CrossRef]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.K.; Charpentier, P.A. Hydroxyapatite–TiO2-based Nanocomposites Synthesized in Supercritical CO2 for Bone Tissue Engineering: Physical and Mechanical Properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef]
- Xia, L.; Xie, Y.; Fang, B.; Wang, X.; Lin, K. In situ modulation of crystallinity and nano-structures to enhance the stability and osseointegration of hydroxyapatite coatings on Ti-6Al-4V implants. Chem. Eng. J. 2018, 347, 711–720. [Google Scholar] [CrossRef]
- Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Chathuranga Senarathna, K.G.; Herath, H.M.T.U.; Premachandra, T.N.; Ranasinghe, C.S.K.; Rajapakse, R.P.V.J.; Rajapakse, R.M.G.; Edirisinghe, M.; Mahalingam, S.; et al. Preparation of bone-implants by coating hydroxyapatite nanoparticles on self-formed titanium dioxide thin-layers on titanium metal surfaces. Mater. Sci. Eng. C 2016, 63, 172–184. [Google Scholar] [CrossRef]
- Visentin, F.; El Habra, N.; Fabrizio, M.; Brianese, N.; Gerbasi, R.; Nodari, L.; Zin, V.; Galenda, A. TiO2-HA bi-layer coatings for improving the bioactivity and service-life of Ti dental implants. Surf. Coat. Technol. 2019, 378, 125049. [Google Scholar] [CrossRef]
- Xu, J.; Aoki, H.; Kasugai, S.; Otsuka, M. Enhancement of mineralization on porous titanium surface by filling with nano-hydroxyapatite particles fabricated with a vacuum spray method. Mater. Sci. Eng. C 2020, 111, 110772. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.H.; Tang, Z.G.; Ramakrishna, S. Development of thin elastomeric composite membranes for biomedical applications. J. Mater. Sci. Mater. Med. 1999, 10, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Raoof, J.B.; Ojani, R. Sensitive electrochemical DNA-based biosensors for the determination of Ag+ and Hg2+ ions and their application in analysis of amalgam filling. J. Iran. Chem. Soc. 2018, 15, 1871–1880. [Google Scholar] [CrossRef]
- Chen, L.L.; Cui, H.F.; Fan, F.; Li, Z.Y.; Han, S.Y.; Ma, X.; Luo, S.W.; Song, X.; Lv, Q.Y. Detection of Helicobacter pylori in dental plaque using a DNA biosensor for noninvasive diagnosis. RSC Adv. 2018, 38, 21075–21083. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gu, W.; Xiao, N.; Ye, L.; Xu, Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int. J. Nanomed. 2014, 9, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef] [Green Version]
- Olteanu, D.; Filip, G.; Socaci, C.; Biris, A.R.; Filip, X.; Coroş, M.; Rosu, M.-C.; Pogacean, F.; Alb, C.; Baldea, I.; et al. Cytotoxicity assessment of graphene-based nanomaterials on human dental follicle stem cells. Colloids Surf. B Biointerfaces 2015, 136, 791–798. [Google Scholar] [CrossRef]
- Song, G.; Guo, X.; Zong, X.; Du, L.; Zhao, J.; Lai, C.; Jin, X. Toxicity of functionalized multi-walled carbon nanotubes on bone mesenchymal stem cell in rats. Dent. Mater. J. 2018, 38, 127–135. [Google Scholar] [CrossRef] [Green Version]




| Material | Main Function | References | |
|---|---|---|---|
| Ca/zeolite | resins | remineralize | [21] |
| Chlorhexidine/zeolite | cement | antimicrobial | [24] |
| Ag/zeolite | cement | antimicrobial | [33,34,35] |
| acrylic resins | antimicrobial | [45] | |
| Zeolite coating | Ti alloys | osseointegration | [49] |
| Restorative Dentistry | Endodontics | Periodontics | Tissue Engineering | Ti Dental Implants | |
|---|---|---|---|---|---|
| Zeolites | Incorporated to fillers when they are loaded with Ca2+, to confer anticaries activity. Ag/zeolite materials can be incorporated to fillers (antibiofilm capacity). | Can incorporate Ca2+ cations and them be incorporated to cements, for enhance their biomineralization activity. Can be loaded with drugs/Ag NPs to confer/enhance antimicrobial/autoinflammatory properties to cements. | Zeolites–HA composites have been described as active in the proliferation of osteoblast. | Coatings to increase osseointegration. Functionalized coatings with antibacterial agents. | |
| Graphene | Reinforcing filler. Ag/GO additive to fillers to improve the antibiofilm capacity. GO quantum dot coatings for dentin hypersensitivity dentin treatments. | Reinforcement of cements. Ag/GO to confer/enhance antimicrobial/anti-inflammatory properties to cements. | GO/HA enhance Ca incorporation. Confer antimicrobial properties when is loading | Scaffolds. Provide suitable environment for cell incorporation and growing factors. Functionalization with proteins/peptides to enhance cell growth. | GO and GO/HA coatings enhance the osseointegration, antibacterial activity and mechanical properties; preventing corrosion. |
| Carbon Nanotubes | Reinforcing filler. | Reinforcement of cements. Incorporation of functionalized nanotubes to cement for drug delivery. | Scaffolds. Provide suitable environment for cell incorporation and growing factors. | Coatings to increase the resistance to corrosion and the osseointegration. | |
| Ti Nanotubes | Coatings to increase the surface roughness in order to improve the osseointegration. Functionalized coatings with proteins/hydroxyapatite to enhance osseointegration. Functionalized coatings with anti-inflammatory or antibacterial agents. | ||||
| Hydroxyapatite/Chitosan nanorods | Enhance the biomineralization of enamel. Fluoridated hydroxyapatite nanorods for caries Enhance the biomineralization of cements and act as reinforcement materials. prevention treatments. | Enhance Ca incorporation. Confer antimicrobial properties when is loading with Zn particles. | Scaffolds. The nano-shape enhanced their osteogenic and cell differentiation potential. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. https://doi.org/10.3390/nano10091770
Bonilla-Represa V, Abalos-Labruzzi C, Herrera-Martinez M, Guerrero-Pérez MO. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials. 2020; 10(9):1770. https://doi.org/10.3390/nano10091770
Chicago/Turabian StyleBonilla-Represa, Victoria, Camilo Abalos-Labruzzi, Manuela Herrera-Martinez, and M. Olga Guerrero-Pérez. 2020. "Nanomaterials in Dentistry: State of the Art and Future Challenges" Nanomaterials 10, no. 9: 1770. https://doi.org/10.3390/nano10091770
APA StyleBonilla-Represa, V., Abalos-Labruzzi, C., Herrera-Martinez, M., & Guerrero-Pérez, M. O. (2020). Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials, 10(9), 1770. https://doi.org/10.3390/nano10091770