Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration
Abstract
:1. Introduction
2. Biomaterials for Electrospinning in Tissue Engineering
2.1. Synthetic and Natural Polymers
2.2. Composite Polymeric Electrospun Fibers
2.3. Decellularized Extracellular Matrix (dECM)-Based Electrospun Fibers
3. Bioactivity and Biofunctionalization of Electrospun Scaffolds
3.1. Bulk Biofunctionalization
3.2. Surface Biofunctionalization and Click Chemistry
- Physical adsorption is a simple approach that involves incubating the scaffold in a solution containing biomolecules. The biomolecules attach onto the scaffold surface owing to surface interactions, e.g., electrostatic forces, van der Waals forces, and hydrogen bonds.
- Chemical immobilization of biomolecules to the surface fibers is realized by the creation of a chemical bonding between functional groups of the components and those of bioactive molecules. Compared to physical adsorption, the covalent surface immobilization of biomolecules results in a more efficient coating; moreover, the bioactive components are retained over a longer period of time, promoting tissue regeneration [75]. In particular, an appropriate choice of polymers—biodegradable or nondegradable—allows the release rate of bioactive components to be controlled.
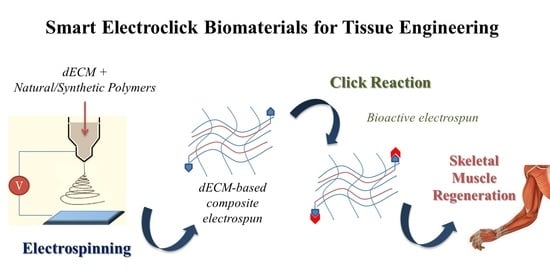
4. Customized Functionalization by Click Chemistry of Composite dECM-Based Electrospun Scaffold for Skeletal Tissue Regeneration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Smoak, M.M.; Mikos, A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio 2020, 7, 100069. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia. Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Carotenuto, F.; Teodori, L.; Maccari, A.M.; Delbono, L.; Orlando, G.; Di Nardo, P. Turning regenerative technologies into treatment to repair myocardial injuries. J. Cell. Mol. Med. 2020, 24, 2704–2716. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Enginnering and Regenerative Medicine Applications. Materials (Basel) 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 2002, 13, 377–383. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Yao, T.; Baker, M.B.; Moroni, L. Strategies to improve nanofibrous scaffolds for vascular tissue engineering. Nanomaterials 2020, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Ramakrishna, S. What Is Next for Electrospinning? Matter 2020, 2, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Min, L.; Pan, H.; Chen, S.; Wang, C.; Wang, N.; Zhang, J.; Cao, Y.; Chen, X.; Hou, X. Recent progress in bio-inspired electrospun materials. Compos. Commun. 2019, 11, 12–20. [Google Scholar] [CrossRef]
- Ameer, J.M.; Anil Kumar, P.R.; Kasoju, N. Strategies to tune electrospun scaffold porosity for effective cell response in tissue engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Maria Giannitelli, S.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 1: Design of experiments. J. Biomed. Mater. Res. Part A 2015, 103, 91–102. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Mozetic, P.; Rainer, A.; Giannitelli, S.M.; Basoli, F.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 2: Regression. J. Biomed. Mater. Res.-Part A 2015, 103, 103–114. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, N.G.; Shin, C.S.; Shin, H. Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Hanumantharao, S.N.; Rao, S. Multi-functional electrospun nanofibers from polymer blends for scaffold tissue engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Swinehart, I.T.; Badylak, S.F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dyn. 2016, 245, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [Green Version]
- Krishtul, S.; Baruch, L.; Machluf, M. Processed Tissue–Derived Extracellular Matrices: Tailored Platforms Empowering Diverse Therapeutic Applications. Adv. Funct. Mater. 2020, 30, 1900386. [Google Scholar] [CrossRef]
- Santschi, M.; Vernengo, A.; Eglin, D.; D’Este, M.; Wuertz-Kozak, K. Decellularized matrix as a building block in bioprinting and electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Shute, J. Glycosaminoglycan and chemokine/growth factor interactions. Handb. Exp. Pharmacol. 2012, 207, 307–324. [Google Scholar] [CrossRef]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; Van Den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Sanyal, R.; Sanyal, A. Reactive and “clickable” electrospun polymeric nanofibers. Polym. Chem. 2015, 6, 3372–3381. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Zhou, T.; Liu, Y.; Leng, J. Shape Memory Polymer Nanofibers and Their Composites: Electrospinning, Structure, Performance, and Applications. Front. Mater. 2015, 2, 62. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Zha, F.; Chen, W.; Zhang, L.; Yu, D. Electrospun natural polymer and its composite nanofibrous scaffolds for nerve tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6,6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res.-Part A 2016, 104, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Joo, W.; Gu, B.K.; Ha, M.Y.; You, S.J.; Chun, H.J. Collagen/poly(D,L-lactic-co-glycolic acid) composite fibrous scaffold prepared by independent nozzle control multi-electrospinning apparatus for dura repair. J. Ind. Eng. Chem. 2018, 66, 430–437. [Google Scholar] [CrossRef]
- Elmashhady, H.H.; Kraemer, B.A.; Patel, K.H.; Sell, S.A.; Garg, K. Decellularized extracellular matrices for tissue engineering applications. Electrospinning 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef]
- Schoen, B.; Avrahami, R.; Baruch, L.; Efraim, Y.; Goldfracht, I.; Elul, O.; Davidov, T.; Gepstein, L.; Zussman, E.; Machluf, M. Electrospun Extracellular Matrix: Paving the Way to Tailor-Made Natural Scaffolds for Cardiac Tissue Regeneration. Adv. Funct. Mater. 2017, 27, 1700427. [Google Scholar] [CrossRef]
- Gao, S.; Guo, W.; Chen, M.; Yuan, Z.; Wang, M.; Zhang, Y.; Liu, S.; Xi, T.; Guo, Q. Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J. Mater. Chem. B 2017, 5, 2273–2285. [Google Scholar] [CrossRef]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.G.; Sell, S.A.; Garg, K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14, 035010. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef] [PubMed]
- McClellan, P.; Landis, W.J. Recent Applications of Coaxial and Emulsion Electrospinning Methods in the Field of Tissue Engineering. Biores. Open Access 2016, 5, 212–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Ang, L.T.; Goh, J.C.H.; Toh, S.L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res.-Part A 2010, 93, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun nanofibers as substrates for cardiomyogenic differentiation of mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 2577–2587. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.; Han, F.; Zhao, J.; Zhao, Y.; Fan, Y.; Yuan, X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Park, K.E.; Kim, B.S.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Basic fibroblast growth factor-encapsulated PCL nano/microfibrous composite scaffolds for bone regeneration. Polymer 2015, 76, 8–16. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Wang, X.F.; Xu, Y.Y.; Qiao, Y.H.; Guo, X.; Wang, D.F.; Li, Q.; Turng, L.S. Polycaprolactone Nanofibers Containing Vascular Endothelial Growth Factor-Encapsulated Gelatin Particles Enhance Mesenchymal Stem Cell Differentiation and Angiogenesis of Endothelial Cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. Ctgf loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.; Lee, E.; Lotz, M.K.; D’Lima, D.D. Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102090. [Google Scholar] [CrossRef]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic Properties of Electrospun Nanofibrous PCL Scaffolds Equipped With Chitosan-Based Nanoreservoirs of Growth Factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembled multicomposite films. Curr. Opin. Colloid Interface Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Jessel, N.; Atalar, F.; Lavalle, P.; Mutterer, J.; Decher, G.; Schaaf, P.; Voegel, J.C.; Ogier, J. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv. Mater. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Asadian, M.; Rashidi, A.; Majidi, M.; Mehrjoo, M.; Emami, B.A.; Tavassoli, H.; Asl, M.P.; Bonakdar, S. Nanofiber protein adsorption affected by electrospinning physical processing parameters. J. Iran. Chem. Soc. 2015, 12, 1089–1097. [Google Scholar] [CrossRef]
- Koh, H.S.; Yong, T.; Chan, C.K.; Ramakrishna, S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008, 29, 3574–3582. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Walkowiak, B.; Campanella, L. Tissue Engineering Between Click Chemistry and Green Chemistry. An Int. J. Hist. Chem. 2019, 3, 29–38. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lancuški, A.; Fort, S.; Bossard, F. Electrospun azido-PCL nanofibers for enhanced surface functionalization by click chemistry. ACS Appl. Mater. Interfaces 2012, 4, 6499–6504. [Google Scholar] [CrossRef]
- Smith Callahan, L.A.; Xie, S.; Barker, I.A.; Zheng, J.; Reneker, D.H.; Dove, A.P.; Becker, M.L. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34, 9089–9095. [Google Scholar] [CrossRef]
- Lin, F.; Yu, J.; Tang, W.; Zheng, J.; Xie, S.; Becker, M.L. Postelectrospinning “click” modification of degradable amino acid-based poly(ester urea) nanofibers. Macromolecules 2013, 46, 9515–9525. [Google Scholar] [CrossRef]
- Kalaoglu-Altan, O.I.; Kirac-Aydin, A.; Sumer Bolu, B.; Sanyal, R.; Sanyal, A. Diels-Alder “clickable” Biodegradable Nanofibers: Benign Tailoring of Scaffolds for Biomolecular Immobilization and Cell Growth. Bioconjug. Chem. 2017, 28, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced schwann cell attachment and alignment using one-pot “Dual Click” GRGDS and YIGSR derivatized nanofibers. Biomacromolecules 2015, 16, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Hua, G.; Yu, J.; Lin, F.; Wade, M.B.; Reneker, D.H.; Becker, M.L. Post-electrospinning “triclick” functionalization of degradable polymer nanofibers. ACS Macro Lett. 2015, 4, 207–213. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix–What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Ma, L.; Yin, X.; Luo, Y.; Yang, H.; Zhang, B. Nano- and Microfabrication for Engineering Native-Like Muscle Tissues. Small Methods 2020, 4, 1900669. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Shayan, M.; Huang, N.F. Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1801168. [Google Scholar] [CrossRef]
- Narayanan, N.; Jiang, C.; Uzunalli, G.; Thankappan, S.K.; Laurencin, C.T.; Deng, M. Polymeric Electrospinning for Musculoskeletal Regenerative Engineering. Regen. Eng. Transl. Med. 2016, 2, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef] [Green Version]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tarnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Porzionato, A.; Sfriso, M.M.; Pontini, A.; Macchi, V.; Petrelli, L.; Pavan, P.G.; Natali, A.N.; Bassetto, F.; Vindigni, V.; De Caro, R. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int. J. Mol. Sci. 2015, 16, 14808–14831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviss, K.J.; Gough, J.E.; Downes, S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef]
- Cooper, A.; Jana, S.; Bhattarai, N.; Zhang, M. Aligned chitosan-based nanofibers for enhanced myogenesis. J. Mater. Chem. 2010, 20, 8904–8911. [Google Scholar] [CrossRef]
- Narayanan, N.; Jiang, C.; Wang, C.; Uzunalli, G.; Whittern, N.; Chen, D.; Jones, O.G.; Kuang, S.; Deng, M. Harnessing Fiber Diameter-Dependent Effects of Myoblasts Toward Biomimetic Scaffold-Based Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.H.; Talovic, M.; Dunn, A.J.; Patel, A.; Vendrell, S.; Schwartz, M.; Garg, K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 2528–2537. [Google Scholar] [CrossRef]
- Aubin, H.; Mas-Moruno, C.; Iijima, M.; Schütterle, N.; Steinbrink, M.; Assmann, A.; Gil, F.J.; Lichtenberg, A.; Pegueroles, M.; Akhyari, P. Customized Interface Biofunctionalization of Decellularized Extracellular Matrix: Toward Enhanced Endothelialization. Tissue Eng.-Part C Methods 2016, 22, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhao, M.; Li, S.; Erasquin, U.J.; Wang, H.; Ren, L.; Chen, C.; Wang, Y.; Cai, C. “Click” immobilization of a VEGF-mimetic peptide on decellularized endothelial extracellular matrix to enhance angiogenesis. ACS Appl. Mater. Interfaces 2014, 6, 8401–8406. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Ju, Y.M.; Kim, I.; Elsangeedy, E.; Lee, J.H.; Yoo, J.J.; Atala, A.; Lee, S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods 2020, 171, 77–85. [Google Scholar] [CrossRef]
- Jazkewitsch, O.; Ritter, H. Formation and characterization of inclusion complexes of alkyne functionalized poly(ε-caprolactone) with β-cyclodextrin. Pseudo-polyrotaxane-based supramolecular organogels. Macromolecules 2011, 44, 375–382. [Google Scholar] [CrossRef]
- Lee, H.J.; Fernandes-Cunha, G.M.; Putra, I.; Koh, W.G.; Myung, D. Tethering Growth Factors to Collagen Surfaces Using Copper-Free Click Chemistry: Surface Characterization and in Vitro Biological Response. ACS Appl. Mater. Interfaces 2017, 9, 23389–23399. [Google Scholar] [CrossRef]
- Syverud, B.C.; VanDusen, K.W.; Larkin, L.M. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs 2016, 202, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, L.; Elsangeedy, E.; Lee, H.; Atala, A.; Yoo, J.; Lee, S.; YM, J. In Vitro Evaluation of Functionalized Decellularized Muscle Scaffold for in Situ Skeletal Muscle Regeneration. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.J.; Kim, J.; Green, J.J. Biomolecule delivery to engineer the cellular microenvironment for regenerative medicine. Ann. Biomed. Eng. 2014, 42, 1557–1572. [Google Scholar] [CrossRef] [PubMed]



| Polymeric Component | Loaded Biomolecules | Method of Preparation | Reference |
|---|---|---|---|
| PLGA | bFGF | Coaxial electrospinning | [63] |
| PVA core PCL shell | GF loaded liposomes | Coaxial electrospinning | [64] |
| PCLC | VEGF | Emulsion electrospinning | [65] |
| PELCL core PELCL shell | VEGF PDGF | Coaxial electrospinning | [66] |
| PCL | bFGF | Emulsion electrospinning | [67] |
| PCL | VEGF | Blend electrospinning | [68] |
| PVA core PLA shell | CTGF | Coaxial electrospinning | [69] |
| PLA | PDGF | Coaxial electrospinning | [70] |
| Electrospun Biomaterials | Experimental Model | Outcomes | Reference |
|---|---|---|---|
| PCL/collagen I | In vitro: Human skeletal muscle cells (hSkMCs) | Aligned PCL/collagen nanofibers significantly induced muscle cell alignment and myotube formation as compared to randomly oriented nanofibers | [47] |
| PLGA | In vitro: Murine myoblast cells (C2C12) | Aligned PLGA fibers control the myoblast elongation and alignment and encourage myoblast differentiation. | [92] |
| Chitosan/PCL | In vitro: Murine myoblast cells (C2C12) | Aligned chitosan-PCL nanofibrous scaffolds exhibited superior tensile strength compared to randomly oriented nanofibers and promoted muscle cell proliferation. | [93] |
| Chitosan/PVA | In vitro: Rabbit’s bone marrow (MSCs) In vivo: Adult New Zealand rabbit | Good cell viability, adhesion growth, and significant proliferation with less immune responses when the scaffold was implanted into the leg muscle of rabbit. | [51] |
| dECM from rabbit skeletal muscle | In vivo: Rabbit | The decellularization protocol of skeletal muscle tissue retains important ECM components. Electrospun scaffold derived completely from skeletal muscle dECM. | [30] |
| PLGA | In vitro: Murine myoblast cells (C2C12) In vivo: Mdx mice | Aligned PLGA fiber with larger diameter support enhanced alignment, growth, and differentiation of myoblasts. In vivo the optimized scaffolds seeded with primary myoblasts result in the formation of dystrophin-positive myofibers network. | [94] |
| PCL/dECM from bovine skeletal muscle | In vitro: Rat muscle precursor cells In vivo: C57/BL6 adult mice | Aligned nanofibers support satellite cell growth, myogenic protein expression, and myokine production. In vivo: myofiber regeneration was observed. | [60,95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politi, S.; Carotenuto, F.; Rinaldi, A.; Di Nardo, P.; Manzari, V.; Albertini, M.C.; Araneo, R.; Ramakrishna, S.; Teodori, L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials 2020, 10, 1781. https://doi.org/10.3390/nano10091781
Politi S, Carotenuto F, Rinaldi A, Di Nardo P, Manzari V, Albertini MC, Araneo R, Ramakrishna S, Teodori L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials. 2020; 10(9):1781. https://doi.org/10.3390/nano10091781
Chicago/Turabian StylePoliti, Sara, Felicia Carotenuto, Antonio Rinaldi, Paolo Di Nardo, Vittorio Manzari, Maria Cristina Albertini, Rodolfo Araneo, Seeram Ramakrishna, and Laura Teodori. 2020. "Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration" Nanomaterials 10, no. 9: 1781. https://doi.org/10.3390/nano10091781
APA StylePoliti, S., Carotenuto, F., Rinaldi, A., Di Nardo, P., Manzari, V., Albertini, M. C., Araneo, R., Ramakrishna, S., & Teodori, L. (2020). Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials, 10(9), 1781. https://doi.org/10.3390/nano10091781