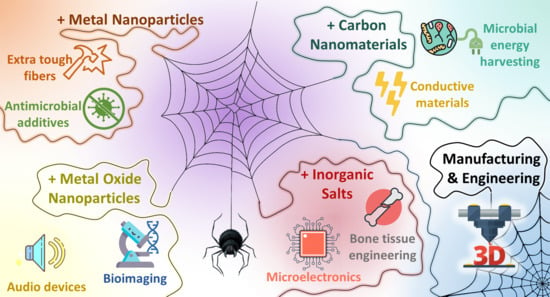
Hybrid Spider Silk with Inorganic Nanomaterials
Abstract
:1. Introduction
2. Spider Silks Characterization and Properties
2.1. Araneomorphae Silk and Its Chemical Composition
- (1)
- The GPGXX unit (X = Q, G, Y), which forms a β-turn spiral structure, is responsible for the exceptional elastic properties of flagelliform silk [35].
- (2)
- An or GAn are alanine-rich motifs containing 6–9 alanine amino acids that form crystalline β-sheets, exceptionally important for tensile strength [36,37]. Dragline silk is especially rich in alanine motifs, which are the main reason for high tensile strength, being the key feature that characterizes draglines [38]. Fragelliform silk in contrast to draglines, does not contain as many alanine units.
- (3)
- GGX unit (X = Y, L, Q) is rich in glycine, which is known by forming 310-helical units and connecting different crystalline regions (stacks of β-sheets). This structure is responsible for the elastic properties of draglines and flagelliform silks [39].
- (4)
- Spacers: contain charged groups and split repetitive peptide units into clusters [40].
- (5)
- NR: non-repetitive regions at the amino- and carboxyl-ends of proteins [41].
| Spinning Gland | Function | Protein | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation (%) | Ref. |
|---|---|---|---|---|---|---|
| Major ampullate gland | Dragline; frame of the web | MaSp1; MaSp2 | 1200 ± 200 | 3.4–11.5 | 25–35 | [49] |
| Minor ampullate gland | Auxiliary spiral threads | MiSp | 900 ± 50 | 3.0 ± 0.6 | 5 | [50] |
| Fragelliform gland | Core fiber for prey capture | Flag | 800 ± 100 | 0.012–0.08 | ≥200 | [51] |
| Cylindrical gland | Outer egg sac | TuSp | 400 ± 50 | 8.7 ± 0.9 | 5–20 | [52] |
| Pyriform gland | Attachment element | PySp | 100 ± 40 | 0.2 ± 0.1 | 50–80 | [53] |
| Aciniform gland | Inner egg sac; prey wrapping | AcSp | 600 ± 50 | 10.4 ± 1.4 | 80 | [47] |
| Aggregate gland | Aqueous coating | AgSp | 800 ± 200 | 1.0 ± 0.1 | 50–100 | [54] |
| Material | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation (%) |
|---|---|---|---|
| Dragline silk | 140–1600 | 3.4–11.5 | 16–350 |
| Bombyx mori (silk) | 500–600 | 9.6 ± 0.6 | 70 |
| Elastin | 2 | 0.001 | 1.6 |
| Kevlar | 3600 | 130 | 60 |
| Nylon 66 | 750–950 | 2–3.6 | 80 |
| High tensile steel | 1650 | 200 ± 10 | 6 |
| Carbon fiber | 4000 | 300 | 25 |
2.2. Mygalomorphae Silk and Its Chemical Composition
2.3. Spider Silk and Silkworm Silk
2.4. Native Spider Silk and Its Recombinant Analogs
3. Modification of Spider Silk by Inorganic Nanomaterials
3.1. Metal Nanoparticles
3.2. Metal Oxide Nanoparticles
3.3. Nanomaterials Based on Inorganic Salts
3.4. Carbon Nanomaterials
4. Trends in Manufacturing Hybrid Silk-Based Materials
5. Future Directions and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. The chemistry of polymer-clay hybrids. Mater. Sci. Eng. C 1995, 3, 109–115. [Google Scholar] [CrossRef]
- Godovski, D.Y. Electron behavior and magnetic properties of polymer nanocomposites. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Silks as ancient models for modern polymers. Polymer 2009, 50, 5623–5632. [Google Scholar] [CrossRef] [Green Version]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Heal. Mater. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.K.T.; Cheung, K.H.Y. Natural Fiber-Reinforced Polymer-Based Composites; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef] [Green Version]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Porter, D.; Guan, J.; Vollrath, F. Spider silk: Super material or thin fibre? Adv. Mater. 2012. [Google Scholar] [CrossRef]
- Singh, K.; Maity, S.; Singha, M. Spinning and applications of spider silk. Front. Sci. 2012, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Vollrath, F. Strength and structure of spiders’ silks. Rev. Mol. Biotechnol. 2000, 74, 67–83. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef]
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural origin of the strain-hardening of spider silk. Adv. Funct. Mater. 2010, 21, 772–778. [Google Scholar] [CrossRef]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Vogt, P.M. Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J. Cell. Mol. Med. 2007. [Google Scholar] [CrossRef] [Green Version]
- Hakimi, O.; Knight, D.P.; Vollrath, F.; Vadgama, P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007, 38, 324–337. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Yarger, J.L.; Cherry, B.R.; van der Vaart, A. Uncovering the structure-function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, A.C.; Sima, Y.H.; Lu, C.; Xiang, Z.H.; Nakagaki, M. The Molecular structures of major ampullate silk proteins of the wasp spider, argiope bruennichi: A second blueprint for synthesizing de novo silk. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 164, 151–158. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, W.; Zhang, J.; Fan, J.S.; Yang, D. Solution structure of eggcase silk protein and its implications for silk fiber formation. Proc. Natl. Acad. Sci. USA 2009, 106, 8906–8911. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef]
- Hu, X.; Vasanthavada, K.; Kohler, K.; McNary, S.; Moore, A.M.F.; Vierra, C.A. Molecular mechanisms of spider silk. Cell. Mol. Life Sci. 2006, 63, 1986–1999. [Google Scholar] [CrossRef]
- Dicko, C.; Vollrath, F.; Kenney, J.M. Spider silk protein refolding is controlled by changing PH. Biomacromolecules 2004, 5, 704–710. [Google Scholar] [CrossRef]
- Hedhammar, M.; Rising, A.; Grip, S.; Martinez, A.S.; Nordling, K.; Casals, C.; Stark, M.; Johansson, J. Structural properties of recombinant nonrepetitive and repetitive parts of major ampullate Spidroin 1 from Euprosthenops Australis: Implications for fiber formation. Biochemistry 2008, 47, 3407–3417. [Google Scholar] [CrossRef]
- Kluge, J.A.; Rabotyagova, O.; Leisk, G.G.; Kaplan, D.L. Spider silks and their applications. Trends Biotechnol. 2008, 26, 244–251. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat. Nanotechnol. 2006, 1, 195–200. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Oppenheim, T.W.; Buell, A.K.; Chirgadze, D.Y.; Welland, M.E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nat. Nanotechnol. 2010, 5, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Rominger, K.M.; Nestor, G.; Eriksson, J.E.; Seisenbaeva, G.A.; Kessler, V.G. Complexes of Keggin POMs [PM12O40]3– (M = Mo, W) with GlyGly Peptide and Arginine—Crystal structures and solution reactivity. Eur. J. Inorg. Chem. 2019, 2019, 4297–4305. [Google Scholar] [CrossRef]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent advances in development of functional spider silk-based hybrid materials. Front. Chem. 2020, 8, 8. [Google Scholar] [CrossRef]
- Lee, S.M.; Pippel, E.; Gösele, U.; Dresbach, C.; Qin, Y.; Chandran, C.V.; Bräuniger, T.; Hause, G.; Knez, M. Greatly increased toughness of infiltrated spider silk. Science 2009, 324, 488–492. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Bittencourt, D.; Dittmar, K.; Lewis, R.V.; Rech, E.L. A MaSp2-like gene found in the Amazon mygalomorph spider Avicularia juruensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Vehoff, T.; Glišović, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical properties of spider dragline silk: Humidity, hysteresis, and relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barghout, J.Y.; Thiel, B.L.; Viney, C. Spider (Araneus diadematus) cocoon silk: A case of non-periodic lattice crystals with a twist? Int. J. Biol. Macromol. 1999, 24, 211–217. [Google Scholar] [CrossRef]
- Brooks, A.E.; Stricker, S.M.; Joshi, S.B.; Kamerzell, T.J.; Middaugh, C.R.; Lewis, R.V. Properties of synthetic spider silk fibers based on Argiope aurantia MaSp2. Biomacromolecules 2008, 9, 1506–1510. [Google Scholar] [CrossRef]
- Lewis, R.V.; Hinman, M.; Kothakota, S.; Fournier, M.J. Expression and purification of a spider silk protein: A new strategy for producing repetitive proteins. Protein Expr. Purif. 1996, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.; Ray, E.; Jelinski, L.W. Solid-state 13C NMR of Nephila clavipes dragline silk establishes structure and identity of crystalline regions. Macromolecules 1994, 27, 35–37. [Google Scholar] [CrossRef]
- Parkhe, A.D.; Seeley, S.K.; Gardner, K.; Thompson, L.; Lewis, R.V. Structural studies of spider silk proteins in the fiber. J. Mol. Recognit. 1997, 10, 1–6. [Google Scholar] [CrossRef]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 71–75. [Google Scholar] [CrossRef]
- Bram, A.; Bränden, C.; Craig, C.; Snigireva, I.; Riekel, C. X-ray diffraction from single fibres of spider silk. J. Appl. Crystallogr. 1997, 30, 90–92. [Google Scholar] [CrossRef]
- Scheibel, T. Spider silks: Recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell Factories 2004, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, R.; Scheibel, T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry 2004, 43, 13604–13612. [Google Scholar] [CrossRef]
- Hinman, M.B.; Jones, J.A.; Lewis, R.V. Synthetic spider silk: A modular fiber. Trends Biotechnol. 2000, 18, 374–379. [Google Scholar] [CrossRef]
- Rising, A.; Nimmervoll, H.; Grip, S.; Fernandez-Arias, A.; Storckenfeldt, E.; Knight, D.P.; Vollrath, F.; Engström, W. Spider silk proteins—Mechanical property and gene sequence. Zool. Sci. 2005, 22, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, C.Y.; Lewis, R.V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998, 275, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Casem, M.L.; Collin, M.A.; Ayoub, N.A.; Hayashi, C.Y. Silk gene transcripts in the developing tubuliform glands of the Western black widow, Latrodectus hesperus. J. Arachnol. 2010, 38, 99–103. [Google Scholar] [CrossRef]
- Blasingame, E.; Tuton-Blasingame, T.; Larkin, L.; Falick, A.M.; Zhao, L.; Fong, J.; Vaidyanathan, V.; Visperas, A.; Geurts, P.; Hu, X.; et al. PyriformSpidroin 1, a novel member of the silk gene family that anchors dragline silk fibers inattachment discs of the black widow spider, Latrodectus hesperus. J. Biol. Chem. 2009, 284, 29097–29108. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, C.Y.; Blackledge, T.A.; Lewis, R.V. Molecular and mechanical characterization of acini-form silk: Uniformity of iterated sequence modules in a novel member of the spider silk fibroingene family. Mol. Biol. Evol. 2004, 21, 1950–1959. [Google Scholar] [CrossRef] [Green Version]
- Agnarsson, I.; Blackledge, T.A. Can a spider web be too sticky? Tensile mechanics constrains the evolution of capture spiral stickiness in orb-weaving spiders. J. Zool. 2009, 278, 134–140. [Google Scholar] [CrossRef]
- Kerr, G.G.; Nahrung, H.F.; Wiegand, A.; Kristoffersen, J.; Killen, P.; Brown, C.; Macdonald, J. Mechanical properties of silk of the Australian golden orb weavers Nephila pilipes and Nephila plumipes. Biol. Open 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Vienneau-Hathaway, J.M.; Brassfield, E.R.; Lane, A.K.; Collin, M.A.; Correa-Garhwal, S.M.; Clarke, T.H.; Schwager, E.E.; Garb, J.E.; Hayashi, C.Y.; Ayoub, N.A. Duplication and concerted evolution of MiSp-encoding genes underlie the material properties of minor ampullate silks of cobweb weaving spiders. BMC Evol. Biol. 2017, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Perea, G.B.; Riekel, C.; Guinea, G.V.; Madurga, R.; Daza, R.; Burghammer, M.; Hayashi, C.; Elices, M.; Plaza, G.R.; Pérez-Rigueiro, J. Identification and dynamics of polyglycine II nanocrystals in Argiope trifasciata flagelliform silk. Sci. Rep. 2013, 3, 3061. [Google Scholar] [CrossRef]
- Van Nimmen, E.; Gellynck, K.; Gheysens, T.; Van Langenhove, L.; Mertens, J. Modeling of the stress-strain behavior of egg sac silk of the spider Araneus diadematus. J. Arachnol. 2005, 33, 629–639. [Google Scholar] [CrossRef]
- Greco, G.; Wolff, J.O.; Pugno, N.M. Strong and tough silk for resilient attachment discs: The mechanical properties of piriform silk in the Spider Cupiennius salei (Keyserling, 1877). Front. Mater. 2020, 7, 138. [Google Scholar] [CrossRef]
- Blackledge, T.A.; Hayashi, C.Y. Unraveling the mechanical properties of composite silk threads spun by cribellate orb-weaving spiders. J. Exp. Biol. 2006, 209, 3131–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, F.A. The role of silk in prey capture by Nonaraneomorph spiders. In Spiders: Webs, Behavior and Evolution; Shear, W.A., Ed.; Stanford University Press: Redwood City, CA, USA, 1986; pp. 269–305. [Google Scholar]
- Palmer, J.M. The silk and silk production system of the funnel-web mygalomorph spider Euagrus (Araneae, Diplurudae). J. Morphol. 1985, 186, 195–207. [Google Scholar] [CrossRef]
- Hajer, J.; Karschová, S.; Řeháková, D. Silks and silk-producing organs of Neotropical tarantula Avicularia metallica (Araneae, Mygalomorphae, Theraphosidae). Ecol. Montenegrina 2016, 7, 313–327. [Google Scholar] [CrossRef]
- Garb, J.E.; diMauro, T.; Lewis, R.V.; Hayashi, C.Y. Expansion and intragenic homogenization of spider silk genes since the Triassic: Evidence from Mygalomorphae (tarantulas and their kin) spidroins. Mol. Biol. Evol. 2007, 24, 2454–2464. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.A.; Garb, J.E.; Edgerly, J.S.; Hayashi, C.Y. Characterization of silk spun by the embiopteran, Antipaluria urichi. Insect Biochem. Mol. Biol. 2009, 39, 75–82. [Google Scholar] [CrossRef]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Numata, K.; Hikima, T.; Guan, J.; Mandal, B.B.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of silk sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Bon, F.X. A discourse upon the usefulness of the silk of spiders. By Monsieur Bon, President of the Court of Accounts, Aydes and Finances, and President of the Royal Society of Science at Montpellier. Communicated by author. Philos. Trans. R. Soc. Lond. 1710, 27, 2–16. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, M.; Keerl, D.; Scheibel, T. Spider silk: From soluble protein to extraordinary fiber. Angew. Chem. 2009, 48, 3584–3596. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Barr, L.A.; Fahnestock, S.R.; Liu, Z.-B. High yield recombinant silk- like protein production in transgenic plants through protein targeting. Transgenic Resour. 2005, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Slotta, U.; Tammer, M.; Kremer, F.; Koelsch, P.; Scheibel, T. Structural analysis of spider silk films. Supramol. Chem. 2006, 18, 465–471. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2006, 82, 223–233. [Google Scholar] [CrossRef]
- Buehler, M.J. Materials by design—A perspective from atoms to structures. MRS Bull. 2013, 38, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Mayes, E.L.; Vollrath, F.; Mann, S.C. Fabrication of magnetic spider silk and other silk-fiber composites using inorganic nanoparticles. Adv. Mater. 1998. [Google Scholar] [CrossRef]
- Singh, A.; Hede, S.; Sastry, M. Spider silk as an active scaffold in the assembly of gold nanoparticles and application of the gold—silk bioconjugate in vapor sensing. Small 2007, 3, 466–473. [Google Scholar] [CrossRef]
- Boutry, C.; Blackledge, T.A. Evolution of supercontraction in spider silk: Structure-function relationship from tarantulas to orb-weavers. J. Exp. Biol. 2010, 213, 3505–3514. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Vollrath, F. The effect of solvents on the contraction and mechanical properties of spider silk. Polymer 1999, 40, 1799–1806. [Google Scholar] [CrossRef]
- Krasteva, N.; Besnard, I.; Guse, B.; Bauer, R.E.; Müllen, K.; Yasuda, A.; Vossmeyer, T. Self-assembled gold nanoparticle/dendrimer composite films for vapor sensing applications. Nano Lett. 2002, 2, 551–555. [Google Scholar] [CrossRef]
- Steven, E.; Park, J.G.; Paravastu, A.; Lopes, E.B.; Brooks, J.S.; Englander, O.; Siegrist, T.; Kaner, P.; Alamo, R.G. Physical characterization of functionalized spider silk: Electronic and sensing properties. Sci. Technol. Adv. Mater. 2011, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, D.; Das, S. Antibacterial activities of cobweb protein. Clin. Microbiol. Infect. 2009. [Google Scholar] [CrossRef] [Green Version]
- Roozbahani, H.; Asmar, M.; Ghaemi, N.; Issazadeh, K. Evaluation of antimicrobial activity of spider silk pholcus phalangioides against two bacterial pathogens in food borne. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2197–2199. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; Beukes, L.S. Cobweb as novel biomaterial for the green and eco-friendly synthesis of silver nanoparticles. Appl. Nanosci. 2016, 6, 863–874. [Google Scholar] [CrossRef] [Green Version]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Azeez, M.A.; Yekeen, T.A.; Akinboro, A. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: Hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J. Clust. Sci. 2017, 28, 1379–1392. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Sazonova, I.Y.; Erdem, S.S.; Hara, T.; Thompson, B.D.; Patel, P.; Botnaru, I.; Lin, C.P.; Reed, G.L.; Weissleder, R.; et al. Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine 2012, 7, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Zhou, J.; Miles, R.N. Sensing fluctuating airflow with spider silk. Proc. Natl. Acad. Sci. USA 2017, 114, 12120–12125. [Google Scholar] [CrossRef] [Green Version]
- Seidel, A.; Liivak, O.; Calve, S.; Adaska, J.; Ji, G.; Yang, Z.; Grubb, D.; Zax, D.B.; Jelinski, L.W. Regenerated spider silk: Processing, properties, and structure. Macromolecules 2000, 33, 775–780. [Google Scholar] [CrossRef]
- Singh, N.; Mondal, D.; Sharma, M.; Devkar, R.V.; Dubey, S.; Prasad, K. Sustainable processing and synthesis of nontoxic and antibacterial magnetic nanocomposite from spider silk in neoteric solvents. ACS Sustain. Chem. Eng. 2015, 3, 2575–2581. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, D.; Yang, M.; Liao, X.; Kang, Y.; Yin, G.; Yao, Y.; Hao, B. Preparation and characterization of the biomineralized zinc oxide particles in spider silk peptides. J. Colloid Interface Sci. 2008, 325, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Pippel, E.; Moutanabbir, O.; Kim, J.H.; Lee, H.J.; Knez, M. In situ raman spectroscopic study of al-infiltrated spider dragline silk under tensile deformation. ACS Appl. Mater. Interfaces 2014. [Google Scholar] [CrossRef]
- Cohen, E.; Moussian, B. Extracellular Composite Matrices in Arthropods; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Kandas, I.; Shehata, N.; Hassounah, I.; Sobolčiak, P. Optical fluorescent spider silk electrospun nanofibers with embedded cerium oxide nanoparticles. J. Nanophotonics 2018, 12, 1. [Google Scholar] [CrossRef]
- Tow, K.H.; Chow, D.M.; Vollrath, F.; Dicaire, I.; Gheysens, T.; Thévenaz, L. Exploring the use of native spider silk as an optical fiber for chemical sensing. J. Lightwave Technol. 2018, 36, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Rufaihah, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef]
- Kiseleva, A.; Kiselev, G.; Kessler, V.; Seisenbaeva, G.; Gets, D.; Rumyantseva, V.; Lyalina, T.; Fakhardo, A.; Krivoshapkin, P.; Krivoshapkina, E. Optically active hybrid materials based on natural spider silk. ACS Appl. Mater. Interfaces 2019, 11, 22962–22972. [Google Scholar] [CrossRef]
- Berman, A.; Hanson, J.; Leiserowitz, L.; Koetzle, T.F.; Weiner, S.; Addadi, L. Biological control of crystal texture: A widespread strategy for adapting crystal properties to function. Science 1993, 259, 776–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Han, J.T.; Cho, K. Formation of amorphous calcium carbonate thin films and their role in biomineralization. Chem. Mater. 2004, 110, 2764–2770. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Fernández-Martínez, A.; Charlet, L.; Tisserand, D.; Renard, F. Textural properties of synthetic nano-calcite produced by hydrothermal carbonation of calcium hydroxide. J. Cryst. Growth 2008, 310, 2946–2953. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Hede, S. Spider silk calcite composite. Hypothesis 2005, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Dmitrović, S.; Jokić, B.; Prekajski, M.; Pantić, J.; Zmejkoski, D.; Zarubica, A.; Matović, B. Synthesis and characterization of spider silk calcite composite. Process. Appl. Ceram. 2016, 10, 37–40. [Google Scholar] [CrossRef]
- Cao, B.; Mao, C. Oriented nucleation of hydroxylapatite crystals on spider dragline silks. Langmuir 2007, 23, 10701–10705. [Google Scholar] [CrossRef]
- Chu, M.; Sun, Y. Self-Assembly method for the preparation of near-infrared fluorescent spider silk coated with cdte nanocrystals. Smart Mater. Struct. 2007, 16, 2453–2456. [Google Scholar] [CrossRef]
- Steven, E.; Saleh, W.R.; Lebedev, V.; Acquah, S.F.A.; Laukhin, V.; Alamo, R.G.; Brooks, J.S. Carbon nanotubes on a spider silk scaffold. Nat. Commun. 2013. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Xie, Y.; Ji, A.; Cao, A.; Fang, Y.; Shi, E. Carbon-nanotube-wrapped spider silks for directed cardiomyocyte growth and electrophysiological detection. ACS Appl. Mater. Interfaces 2018, 10, 6793–6798. [Google Scholar] [CrossRef]
- Lepore, E.; Bosia, F.; Bonaccorso, F.; Bruna, M.; Taioli, S.; Garberoglio, G.; Ferrari, A.C.; Pugno, N.M. Spider silk reinforced by graphene or carbon nanotubes. 2D Mater. 2017, 4, 031013. [Google Scholar] [CrossRef]
- Firm, T.H.E.; Karlf, O.F. Method for Separating Trivalent Americium from Trivalent Curium. U.S. Patent 2007/0009410A1, 11 January 2007. [Google Scholar]
- Bosia, F.; Buehler, M.J.; Pugno, N.M. Hierarchical simulations for the design of supertough nanofibers inspired by spider silk. Phys. Rev. E 2010, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, P.; Cai, X.; Zhou, S.; Yuan, Y. Naturally derived carbon nanofibers as sustainable electrocatalysts for microbial energy harvesting: A new application of spider silk. Appl. Catal. B Environ. 2016, 188, 31–38. [Google Scholar] [CrossRef]
- Porter, D.; Vollrath, F. Silk as a biomimetic ideal for structural polymers. Adv. Mater. 2009, 21, 487–492. [Google Scholar] [CrossRef]
- Wu, Y.; Shah, D.U.; Liu, C.; Yu, Z.; Liu, J.; Ren, X.; Rowland, M.J.; Abell, C.; Ramage, M.H.; Scherman, O.A. Bioinspired supramolecular fibers drawn from a multiphase self-assembled hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 8163–8168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, S.; Zhao, S.; Muth, C.; Paez, J.; del Campo, A. Toward light-regulated living biomaterials. Adv. Sci. 2018, 5, 1800383. [Google Scholar] [CrossRef]
- Mu, X.; Fitzpatrick, V.; Kaplan, D. From silk spinning to 3D printing: Polymer manufacturing using directed hierarchical molecular assembly. Adv. Healthc. Mater. 2020, 9, 1901552. [Google Scholar] [CrossRef]
- DeSimone, E.; Jungst, T.; Schacht, K.; Groll, J.; Scheibel, T. Biofabrication of 3D constructs: Fabrication technologies and spider silk proteins as bioinks. Pure Appl. Chem. 2015, 87, 737–749. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseleva, A.P.; Kiselev, G.O.; Nikolaeva, V.O.; Seisenbaeva, G.; Kessler, V.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Hybrid Spider Silk with Inorganic Nanomaterials. Nanomaterials 2020, 10, 1853. https://doi.org/10.3390/nano10091853
Kiseleva AP, Kiselev GO, Nikolaeva VO, Seisenbaeva G, Kessler V, Krivoshapkin PV, Krivoshapkina EF. Hybrid Spider Silk with Inorganic Nanomaterials. Nanomaterials. 2020; 10(9):1853. https://doi.org/10.3390/nano10091853
Chicago/Turabian StyleKiseleva, Aleksandra P., Grigorii O. Kiselev, Valeria O. Nikolaeva, Gulaim Seisenbaeva, Vadim Kessler, Pavel V. Krivoshapkin, and Elena F. Krivoshapkina. 2020. "Hybrid Spider Silk with Inorganic Nanomaterials" Nanomaterials 10, no. 9: 1853. https://doi.org/10.3390/nano10091853
APA StyleKiseleva, A. P., Kiselev, G. O., Nikolaeva, V. O., Seisenbaeva, G., Kessler, V., Krivoshapkin, P. V., & Krivoshapkina, E. F. (2020). Hybrid Spider Silk with Inorganic Nanomaterials. Nanomaterials, 10(9), 1853. https://doi.org/10.3390/nano10091853