Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Dye–MMT Interaction: Experimental Analysis of Optical Properties
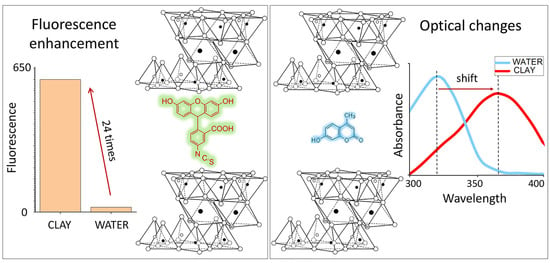
3.1.1. Absorbance and Fluorescent Spectra
3.1.2. Fluorescent Enhancement of Free and Conjugated Dye–MMT Hybrids
3.1.3. Fluorescent Enhancement: Evaluation of the Possible Mechanisms Involved
3.2. Proposed Applications of Free and Conjugated Dye–MMT Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wimpenny, J. Clay Minerals. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer International Publishing: Cham, Germany, 2018; pp. 265–275. ISBN 978-3-319-39312-4. [Google Scholar]
- Segad, M.; Jönsson, B.; Åkesson, T.; Cabane, B. Ca/Na Montmorillonite: Structure, Forces and Swelling Properties. Langmuir 2010, 26, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- El Ouardi, M.; Laabd, M.; Oualid, H.A.; Brahmi, Y.; Abaamrane, A.; Elouahli, A.; Addi, A.A.; Laknifli, A. Efficient removal of p-nitrophenol from water using montmorillonite clay: Insights into the adsorption mechanism, process optimization, and regeneration. Environ. Sci. Pollut. Res. 2019, 26, 19615–19631. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, S.E.; Chinellato, A.C.; Boesel, L.F.; Pessan, L.A. Poly (ethylene terephthalate)-organoclay nanocomposites: Morphological, thermal and barrier properties. J. Metastable Nanocrystalline Mater. 2004, 22, 57–64. [Google Scholar] [CrossRef]
- Bandera, D.; Meyer, V.; Prevost, D.; Zimmermann, T.; Boesel, L. Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications. Polymers 2016, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Thue, P.S.; Sophia, A.C.; Lima, E.C.; Wamba, A.G.N.; de Alencar, W.S.; dos Reis, G.S.; Rodembusch, F.S.; Dias, S.L.P. Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J. Clean. Prod. 2018, 171, 30–44. [Google Scholar] [CrossRef]
- Sadri, S.; Johnson, B.B.; Ruyter-Hooley, M.; Angove, M.J. The adsorption of nortriptyline on montmorillonite, kaolinite and gibbsite. Appl. Clay Sci. 2018, 165, 64–70. [Google Scholar] [CrossRef]
- Ogawa, M.; Takee, R.; Okabe, Y.; Seki, Y. Bio-geo hybrid pigment; clay-anthocyanin complex which changes color depending on the atmosphere. Dye. Pigment. 2017, 139, 561–565. [Google Scholar] [CrossRef]
- Goto, T.; Ogawa, M. Visible-Light-Responsive Photocatalytic Flow Reactor Composed of Titania Film Photosensitized by Metal Complex-Clay Hybrid. ACS Appl. Mater. Interface 2015, 7, 12631–12634. [Google Scholar] [CrossRef]
- Teixeira-Neto, Â.A.; Izumi, C.M.S.; Temperini, M.L.A.; Ferreira, A.M.; Constantino, V.R.L. Hybrid Materials Based on Smectite Clays and Nutraceutical Anthocyanins from the Açaí Fruit. Eur. J. Inorg. Chem. 2012, 2012, 5411–5420. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, Y.; Bai, H.; Huang, M.; Zhang, T.; Song, S. High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ. Pollut. 2020, 257, 113574. [Google Scholar] [CrossRef]
- Kohno, Y.; Kinoshita, R.; Ikoma, S.; Yoda, K.; Shibata, M.; Matsushima, R.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Stabilization of natural anthocyanin by intercalation into montmorillonite. Appl. Clay Sci. 2009, 42, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.L.; de Oliveira, A.V.; de Brito, E.S.; Ribeiro, P.R.; Azeredo, H.M. Stabilizing effect of montmorillonite on acerola juice anthocyanins. Food Chem. 2018, 245, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.M.; Silva, C.P.; Gehlen, M.H.; Oake, J.; Bohne, C.; Quina, F.H. Organic/inorganic hybrid pigments from flavylium cations and palygorskite. Appl. Clay Sci. 2018, 162, 478–486. [Google Scholar] [CrossRef]
- Belbel, A.; Kharroubi, M.; Janot, J.-M.; Abdessamad, M.; Haouzi, A.; Lefkaier, I.K.; Balme, S. Preparation and characterization of homoionic montmorillonite modified with ionic liquid: Application in dye adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 219–227. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Kureková, V.B.; Belušáková, S.; Boháč, P.; Bujdák, J. Resonance energy transfer in the systems of smectite modified with a fluorescent cationic polymer and a photosensitizer. Appl. Clay Sci. 2019, 183, 105326. [Google Scholar] [CrossRef]
- Takagi, S.; Shimada, T.; Ishida, Y.; Fujimura, T.; Masui, D.; Tachibana, H.; Eguchi, M.; Inoue, H. Size-Matching Effect on Inorganic Nanosheets: Control of Distance, Alignment, and Orientation of Molecular Adsorption as a Bottom-Up Methodology for Nanomaterials. Langmuir 2013, 29, 2108–2119. [Google Scholar] [CrossRef]
- Čeklovský, A.; Czímerová, A.; Lang, K.; Bujdák, J. Effect of the layer charge on the interaction of porphyrin dyes in layered silicates dispersions. J. Lumin. 2009, 129, 912–918. [Google Scholar] [CrossRef]
- Bujdák, J. The effects of layered nanoparticles and their properties on the molecular aggregation of organic dyes. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 108–133. [Google Scholar] [CrossRef]
- Fournier, F.; de Viguerie, L.; Balme, S.; Janot, J.-M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Marry, V.; Rotenberg, B.; Turq, P. Structure and dynamics of water at a clay surface from molecular dynamics simulation. Phys. Chem. Chem. Phys. 2008, 10, 4802–4813. [Google Scholar] [CrossRef] [PubMed]
- Katti, D.R.; Katti, K.S.; Raviprasad, M.; Gu, C. Role of Polymer Interactions with Clays and Modifiers on Nanomechanical Properties and Crystallinity in Polymer Clay Nanocomposites. J. Nanomater. 2012, 2012, 341056. [Google Scholar] [CrossRef] [Green Version]
- Heinz, H.; Vaia, R.A.; Krishnamoorti, R.; Farmer, B.L. Self-Assembly of Alkylammonium Chains on Montmorillonite: Effect of Chain Length, Head Group Structure, and Cation Exchange Capacity. Chem. Mater. 2007, 19, 59–68. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer−Clay Nanocomposites Based on Chitosan Intercalated in Montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Yao, H.-B.; Tan, Z.-H.; Fang, H.-Y.; Yu, S.-H. Artificial Nacre-like Bionanocomposite Films from the Self-Assembly of Chitosan–Montmorillonite Hybrid Building Blocks. Angew. Chem. Int. Ed. 2010, 49, 10127–10131. [Google Scholar] [CrossRef]
- Boesel, L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef]
- Matejdes, M.; Czímerová, A.; Janek, M. Fluorescence tuning of 2D montmorillonite optically active layers with beta-cyclodextrine/dye supramolecular complexes. Appl. Clay Sci. 2015, 114, 9–19. [Google Scholar] [CrossRef]
- Sas, S.; Danko, M.; Bizovská, V.; Lang, K.; Bujdák, J. Highly luminescent hybrid materials based on smectites with polyethylene glycol modified with rhodamine fluorophore. Appl. Clay Sci. 2017, 138, 25–33. [Google Scholar] [CrossRef]
- Mikheev, Y.A.; Guseva, L.N.; Ershov, Y.A. The optical properties of molecules and chromogenic aggregates of xanthene dyes. Russ. J. Phys. Chem. A 2009, 83, 792–797. [Google Scholar] [CrossRef]
- Belot, V.; Farran, D.; Jean, M.; Albalat, M.; Vanthuyne, N.; Roussel, C. Steric Scale of Common Substituents from Rotational Barriers of N-(o-Substituted aryl)thiazoline-2-thione Atropisomers. J. Org. Chem. 2017, 82, 10188–10200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangaraj, V.; Janot, J.-M.; Jaber, M.; Bechelany, M.; Balme, S. Adsorption and photophysical properties of fluorescent dyes over montmorillonite and saponite modified by surfactant. Chemosphere 2017, 184, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakato, T.; Nakano, Y.; Mouri, E. Effects of sol–gel transition of clay colloids on the spectroscopic behavior of cationic dye adsorbed on the clay particles. Appl. Clay Sci. 2015, 118, 29–37. [Google Scholar] [CrossRef]
- Nad, S.; Pal, H. Unusual Photophysical Properties of Coumarin-151. J. Phys. Chem. A 2001, 105, 1097–1106. [Google Scholar] [CrossRef]
- Pal, H.; Nad, S.; Kumbhakar, M. Photophysical properties of coumarin-120: Unusual behavior in nonpolar solvents. J. Chem. Phys. 2003, 119, 443–452. [Google Scholar] [CrossRef]
- Ditkovich, J.; Mukra, T.; Pines, D.; Huppert, D.; Pines, E. Bifunctional Photoacids: Remote Protonation Affecting Chemical Reactivity. J. Phys. Chem. B 2014, 119, 2690–2701. [Google Scholar] [CrossRef]
- Xiao, D.; Prémont-Schwarz, M.; Nibbering, E.T.J.; Batista, V.S. Ultrafast Vibrational Frequency Shifts Induced by Electronic Excitations: Naphthols in Low Dielectric Media. J. Phys. Chem. A 2011, 116, 2775–2790. [Google Scholar] [CrossRef]
- Pines, E.; Pines, D.; Gajst, O.; Huppert, D. Reversible intermolecular-coupled-intramolecular (RICI) proton transfer occurring on the reaction-radius a of 2-naphthol-6,8-disulfonate photoacid. J. Chem. Phys. 2020, 152, 074205. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral properties of substituted coumarins in solution and polymer matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Micali, N.; Romeo, A.; Scolaro, L.M. Fractal aggregation of dyes such as porphyrins and related compounds under stacking. Curr. Opin. Colloid Interface Sci. 2000, 5, 49–55. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Čeklovský, A.; Takagi, S.; Bujdák, J. Study of spectral behaviour and optical properties of cis/trans-bis(N-methylpyridinium-4-yl)diphenyl porphyrin adsorbed on layered silicates. J. Colloid Interface Sci. 2011, 360, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Belušáková, S.; Sola-Llano, R.; Lopez Arbeloa, I.; Martínez-Martínez, V.; Bujdák, J. Resonance energy transfer between dye molecules in hybrid films of a layered silicate, including the effect of dye concentration thereon. Appl. Clay Sci. 2018, 155, 57–64. [Google Scholar] [CrossRef]
- Preat, J.; Jacquemin, D.; Wathelet, V.; André, J.-M.; Perpète, E.A. TD-DFT Investigation of the UV Spectra of Pyranone Derivatives. J. Phys. Chem. A 2006, 110, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-Y.; Yang, F.; Hu, G.-B.; Yang, Y.; Huang, W.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Restriction of intramolecular motions (RIM) by metal-organic frameworks for electrochemiluminescence enhancement:2D Zr12-adb nanoplate as a novel ECL tag for the construction of biosensing platform. Biosens. Bioelectron. 2020, 155, 112099. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.; Yu, G.; Sze Lee, P.P.; Liu, Y.; Zhu, D. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Tokieda, D.; Tsukamoto, T.; Ishida, Y.; Ichihara, H.; Shimada, T.; Takagi, S. Unique fluorescence behavior of dyes on the clay minerals surface: Surface Fixation Induced Emission (S-FIE). J. Photochem. Photobiol. A Chem. 2017, 339, 67–79. [Google Scholar] [CrossRef]
- Nakazato, R.; Shimada, T.; Ohtani, Y.; Ishida, T.; Takagi, S. Adsorption and emission enhancement behavior of 4,4′-bipyridine on dispersed montmorillonite nano-sheets under aqueous conditions. Tetrahedron Lett. 2018, 59, 2459–2462. [Google Scholar] [CrossRef]
- Rodriguez-Serrano, A.; Rai-Constapel, V.; Daza, M.C.; Doerr, M.; Marian, C.M. Internal heavy atom effects in phenothiazinium dyes: Enhancement of intersystem crossing via vibronic spin-orbit coupling. Phys. Chem. Chem. Phys. 2015, 17, 11350–11358. [Google Scholar] [CrossRef] [Green Version]
- Bolton, O.J. The Directed Heavy Atom Effect: A Design Principle for Metal-Free Organic Phosphors. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2010. [Google Scholar]
- Giovannini, G.; Hall, A.J.; Gubala, V. Coumarin-based, switchable fluorescent substrates for enzymatic bacterial detection. Talanta 2018, 188, 448–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yang, Y.; Qi, X.; Zhou, X.; Ren, W.X.; Deng, M.; Wu, J.; Lü, M.; Liang, S.; Teichmann, A.T. Design and applications of a novel fluorescent probe for detecting glutathione in biological samples. Anal. Chim. Acta 2020, 1117, 18–24. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Wang, C.; Kong, X.; Tang, Y.; Lin, W. Dual Site-Controlled and Lysosome-Targeted Intramolecular Charge Transfer–Photoinduced Electron Transfer–Fluorescence Resonance Energy Transfer Fluorescent Probe for Monitoring pH Changes in Living Cells. Anal. Chem. 2016, 88, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Giustetto, R.; Wahyudi, O. Sorption of red dyes on palygorskite: Synthesis and stability of red/purple Mayan nanocomposites. Microporous Mesoporous Mater. 2011, 142, 221–235. [Google Scholar] [CrossRef]
- Han, D.; Yi, J.; Liu, C.; Liang, L.; Huang, K.; Jing, L.; Qin, D. A fluoran-based viscosity probe with high-performance for lysosome-targeted fluorescence imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118405. [Google Scholar] [CrossRef]
- Tang, J.; Feng, D.; Yang, J.; Ma, X.; Wang, X.-Q. A turn-on luminescent probe for Fe3+ and ascorbic acid with logic gate operation based on a zinc(ii)-based metal–organic framework. New J. Chem. 2020, 44, 8728–8735. [Google Scholar] [CrossRef]







| E.F. | Chi5:1_FITC | Chi5:2.5_FITC | Chi5:5_FITC | |||
|---|---|---|---|---|---|---|
| mg/mL | 10C | 1C | 10C | 1C | 10C | 1C |
| 1 | 12 | 17 | 37 | 56 | 28 | 17 |
| 0.1 | 5.7 | 4.3 | 17 | 16 | 12 | 16 |
| 0.01 | 2.6 | 2.2 | 1.7 | 1.7 | 4.4 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, G.; Rossi, R.M.; Boesel, L.F. Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications. Nanomaterials 2021, 11, 197. https://doi.org/10.3390/nano11010197
Giovannini G, Rossi RM, Boesel LF. Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications. Nanomaterials. 2021; 11(1):197. https://doi.org/10.3390/nano11010197
Chicago/Turabian StyleGiovannini, Giorgia, René M. Rossi, and Luciano F. Boesel. 2021. "Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications" Nanomaterials 11, no. 1: 197. https://doi.org/10.3390/nano11010197
APA StyleGiovannini, G., Rossi, R. M., & Boesel, L. F. (2021). Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications. Nanomaterials, 11(1), 197. https://doi.org/10.3390/nano11010197