Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis Procedure
2.3. Characterization
3. Results
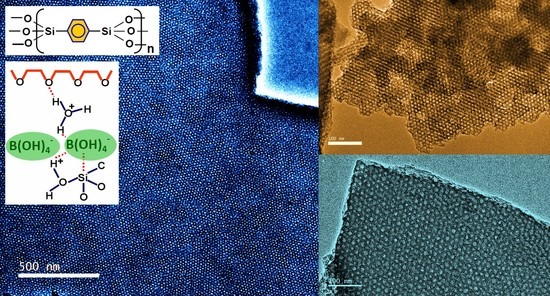
3.1. TEM Images
3.2. SAXS Patterns
3.3. FE-SEM Images
3.4. N2 Adsorption-Desorption Analysis
3.5. Elemental Analysis
3.6. 29Si CP-MAS NMR
3.7. TG-DTA Thermogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, Y.; Shi, Y.; Zhao, D. Designed synthesis of mesoporous solids via nonionic-surfactant-templating approach. Chem. Commun. 2007, 9, 897–926. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Teng, Z. Facile method to efficiently fabricate large-size mesoporous organosilica nanosheets with uniform tunable pore size for robust separation membranes. Chem. Mater. 2019, 31, 3823–3830. [Google Scholar] [CrossRef]
- Choi, E.; Cho, E.-B.; Jaroniec, M. Preparation of highly ordered mesoporous ethane–silicas under weakly acidic conditions and their hydrothermal stability. J. Mater. Chem. A 2017, 5, 21378–21388. [Google Scholar] [CrossRef]
- Nagamune, N.; Ryohei, M. The effect of added inorganic salts on the micelle formation of nonionic surfactants in aqueous solutions. Bull. Chem. Soc. Jpn. 1977, 50, 1690–1694. [Google Scholar]
- Muramoto, N.; Sugiyama, T.; Matsuno, T.; Wada, H.; Kuroda, K.; Shimojima, A. Preparation of periodic mesoporous organosilica with large mesopores using silica colloidal crystals as templates. Nanoscale 2020, 12, 21155–21164. [Google Scholar] [CrossRef] [PubMed]
- Kipkemboi, P.; Fogden, A.; Alfredsson, V.; Flodström, K. Triblock copolymers as templates in mesoporous silica formation: Structural dependence on polymer chain length and synthesis temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Pal, N.; Sim, S.; Cho, E.-B. Multifunctional periodic mesoporous benzene-silicas for evaluation of CO2 adsorption at standard temperature and pressure. Microporous Mesoporous Mater. 2020, 293, 109816. [Google Scholar] [CrossRef]
- Lee, G.; Choi, E.; Yang, S.; Cho, E.-B. Tailoring pore size, structure, and morphology of hierarchical mesoporous silica using diblock and pentablock copolymer templates. J. Phys. Chem. C 2018, 122, 4507–4516. [Google Scholar] [CrossRef]
- Li, Y.S.; Shi, J.L.; Hua, Z.L.; Chen, H.R.; Ruan, M.L.; Yan, D.S. Hollow spheres of mesoporous aluminosilicate with a three-dimensional pore network and extraordinarily high hydrothermal stability. Nano Lett. 2003, 3, 609–612. [Google Scholar] [CrossRef]
- Kuang, D.B.; Brezesinski, T.; Smarsly, B. Hierarchical porous silica materials with a trimodal pore system using surfactant templates. J. Am. Chem. Soc. 2004, 126, 10534–10535. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Brunella, V.; Berlier, G.; Ugazio, E.; Scalarone, D. Effect of multimodal pore channels on cargo release from mesoporous silica nanoparticles. J. Nanomater. 2016, 2016, 1325174. [Google Scholar]
- Cho, E.-B.; Kim, D.; Mandal, M.; Gunathilake, C.A.; Jaroniec, M. Benzene-silica with hexagonal and cubic ordered mesostructures synthesized in the presence of block copolymers and weak acid catalysts. J. Phys. Chem. C 2012, 116, 16023–16029. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, X.; Cui, X. A two-step route to synthesis of small-pored and thick-walled SBA-16-type mesoporous silica under mildly acidic conditions. J. Colloid Interface Sci. 2007, 307, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-B.; Kim, D.; Górka, J.; Jaroniec, M. Periodic mesoporous benzene- and thiophene-silicas prepared using aluminum chloride as an acid catalyst: Effect of aluminum salt/organosilane ratio and stirring time. J. Phys. Chem. C 2009, 113, 5111–5119. [Google Scholar] [CrossRef]
- Cho, E.-B.; Mandal, M.; Jaroniec, M. Periodic mesoporous benzene-silicas prepared using boric acid as catalyst. Chem. Mater. 2011, 23, 1971–1976. [Google Scholar] [CrossRef]
- Seong, H.; Cho, E.-B.; Oh, J.; Chang, T. Synthesis and micellar characterization of CBABC type PLGA-PEO-PPO-PEO-PLGA pentablock copolymers. Bull. Korean Chem. Soc. 2014, 35, 2342–2348. [Google Scholar] [CrossRef] [Green Version]
- Jaroniec, M.; Solovyov, L.A. Improvement of the Kruk-Jaroniec-Sayari method for pore size analysis of ordered silicas with cylindrical mesopores. Langmuir 2006, 22, 6757–6760. [Google Scholar] [CrossRef]
- Xie, W.; Hu, L.; Yang, X. Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for biodiesel production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. J. Chem. Soc. Chem. Commun. 1993, 8, 680–682. [Google Scholar] [CrossRef]
- Pal, N.; Cho, E.-B.; Kim, D. Synthesis of ordered mesoporous silica/ceria–silica composites and their high catalytic performance for solvent-free oxidation of benzyl alcohol at room temperature. RSC Adv. 2014, 4, 9213–9222. [Google Scholar] [CrossRef]
- Ballem, M.A.; Córdoba, J.M.; Odén, M. Influence of synthesis temperature on morphology of SBA-16 mesoporous materials with a three-dimensional pore system. Microporous Mesoporous Mater. 2010, 129, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Mesa, M.; Sierra, L.; Patarin, J.; Guth, J.-L. Morphology and porosity characteristics control of SBA-16 mesoporous silica. Effect of the triblock surfactant Pluronic F127 degradation during the synthesis. Solid State Sci. 2005, 7, 990–997. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Tanev, P.T.; Chibwe, M.; Pinnavaia, T.J. Titanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic compounds. Nature 1994, 368, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.; Carcel, C.; Oliviero, E.; Toquer, G.; Trens, P.; Bartlett, J.R.; Wong, M.; Man, C. Single-template periodic mesoporous organosilica with organized bimodal mesoporosity. Microporous Mesoporous Mater. 2020, 297, 110042. [Google Scholar] [CrossRef]
- Baù, L.; Bártová, B.; Arduini, M.; Mancin, F. Surfactant-free synthesis of mesoporous and hollow silica nanoparticles with an inorganic template. Chem. Commun. 2009, 48, 7584–7586. [Google Scholar] [CrossRef] [PubMed]












| Sample | 1H NMR | GPC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Repeating Unit | Molecular Formula | Mn (g/mol) | wLG | Mn (g/mol) | Mw (g/mol) | PDI | ||||
| PO | EO | LA | GA | |||||||
| PLGF108-220 | 54 | 282 | 44 | 14 | (LA22GA7)EO141PO54EO141(LA22GA7) | 19,462 | 0.201 | 11,100 | 15,000 | 1.35 |
| PLGF108-225 | 54 | 282 | 57 | 18 | (LA28GA9)EO141PO54EO141(LA28GA9) | 20,630 | 0.247 | 10,800 | 15,000 | 1.40 |
| A | |||||
| Sample | Template | TEOS (g) | Ethanol (g) | H2O (g) | Acid Concentration |
| PMSF-1 | PLGF108-220 | 0.8 | 10 | 60 | FeCl3·6H2O/TEOS = 2 |
| PMSF-2 | PLGF108-225 | 0.8 | 10 | 60 | FeCl3·6H2O/TEOS = 2 |
| PMSA-1 | PLGF108-220 | 1.2 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-2 | PLGF108-220 | 0.8 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-3 | PLGF108-220 | 0.4 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-4 | PLGF108-225 | 1.2 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-5 | PLGF108-225 | 0.8 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSA-6 | PLGF108-225 | 0.4 | 10 | 60 | AlCl3·6H2O/TEOS = 2 |
| PMSB-1 | PLGF108-220 | 1.2 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-2 | PLGF108-220 | 0.8 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-3 | PLGF108-220 | 0.4 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-4 | PLGF108-225 | 1.2 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-5 | PLGF108-225 | 0.8 | 10 | 60 | H3BO3/TEOS = 2 |
| PMSB-6 | PLGF108-225 | 0.4 | 10 | 60 | H3BO3/TEOS = 2 |
| B | |||||
| Sample | Template | BTEB (g) | Ethanol (g) | H2O (g) | Acid concentration |
| PMOF-1 | PLGF108-220 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOF-2 | PLGF108-220 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOF-3 | PLGF108-225 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOF-4 | PLGF108-225 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOA-1 | PLGF108-220 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOA-2 | PLGF108-220 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOA-3 | PLGF108-225 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOA-4 | PLGF108-225 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOB-1 | PLGF108-220 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOB-2 | PLGF108-220 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| PMOB-3 | PLGF108-225 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOB-4 | PLGF108-225 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| C | |||||
| Sample | Template | BTEB (g) | Ethanol (g) | H2O (g) | Acid concentration |
| PMOFH-1 | PLGF108-220 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOFH-2 | PLGF108-220 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOFH-3 | PLGF108-225 | 1.8 | 10 | 60 | FeCl3·6H2O/BTEB = 2 |
| PMOFH-4 | PLGF108-225 | 1.0 | 10 | 60 | FeCl3·6H2O/BTEB = 1 |
| PMOAH-1 | PLGF108-220 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOAH-2 | PLGF108-220 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOAH-3 | PLGF108-225 | 1.8 | 10 | 60 | AlCl3·6H2O/BTEB = 2 |
| PMOAH-4 | PLGF108-225 | 1.0 | 10 | 60 | AlCl3·6H2O/BTEB = 1 |
| PMOBH-1 | PLGF108-220 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOBH-2 | PLGF108-220 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| PMOBH-3 | PLGF108-225 | 1.8 | 10 | 60 | H3BO3/BTEB = 2 |
| PMOBH-4 | PLGF108-225 | 1.0 | 10 | 60 | H3BO3/BTEB = 1 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMSA-2 | 755 | 1.03 | 1.01 | 0.87 | 9.50 | 13.81 |
| PMSA-4 | 731 | 0.76 | 0.75 | 0.69 | 3.64, 8.08 | 12.69 |
| PMSB-2 | 737 | 0.69 | 0.61 | 0.51 | 5.69 | 16.11 |
| PMSB-3 | 836 | 0.81 | 0.74 | 0.59 | 2.89, 6.58 | 17.70 |
| PMSB-4 | 388 | 0.74 | 0.52 | 0.21 | 5.45 | 15.14 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMOA-1 | 457 | 0.32 | 0.22 | 0.09 | 5.27 | 19.04 |
| PMOA-2 | 630 | 0.63 | 0.51 | 0.33 | 6.57 | 19.95 |
| PMOA-3 | 691 | 0.49 | 0.31 | 0.19 | 5.72 | 19.63 |
| PMOA-4 | 659 | 0.78 | 0.61 | 0.32 | 6.56 | 19.95 |
| PMOB-1 | 320 | 0.27 | 0.26 | 0.19 | 5.74 | 17.95 |
| PMOB-2 | 578 | 0.50 | 0.41 | 0.35 | 5.72 | 18.21 |
| PMOB-3 | 437 | 0.33 | 0.25 | 0.27 | 5.74 | 17.70 |
| PMOB-4 | 484 | 0.42 | 0.36 | 0.29 | 5.48 | 18.48 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | DKJS (nm) | d (nm) |
| PMOBH-1 | 339 | 0.27 | 0.20 | 0.20 | 4.87, 8.93 | 17.45 |
| PMOBH-2 | 361 | 0.36 | 0.31 | 0.23 | 5.49 | 18.48 |
| PMOBH-3 | 337 | 0.28 | 0.21 | 0.20 | 5.50, 11.24 | 21.30 |
| PMOBH-4 | 210 | 0.39 | 0.26 | 0.03 | 6.00, 13.66 | 23.27 |
| Sample | Al (ppm) | B (ppm) |
|---|---|---|
| PMOA-2 | 665.4 | - |
| PMOA-4 | 650.3 | - |
| PMOB-1 | - | 46.68 |
| PMOB-3 | - | 20.45 |
| PMOBH-1 | - | 142.25 |
| PMOBH-3 | - | 168.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, N.; Sunwoo, Y.; Park, J.-S.; Kim, T.; Cho, E.-B. Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials 2021, 11, 2522. https://doi.org/10.3390/nano11102522
Pal N, Sunwoo Y, Park J-S, Kim T, Cho E-B. Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials. 2021; 11(10):2522. https://doi.org/10.3390/nano11102522
Chicago/Turabian StylePal, Nabanita, Young Sunwoo, Jae-Seo Park, Taeyeon Kim, and Eun-Bum Cho. 2021. "Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media" Nanomaterials 11, no. 10: 2522. https://doi.org/10.3390/nano11102522
APA StylePal, N., Sunwoo, Y., Park, J. -S., Kim, T., & Cho, E. -B. (2021). Newly Designed Mesoporous Silica and Organosilica Nanostructures Based on Pentablock Copolymer Templates in Weakly Acidic Media. Nanomaterials, 11(10), 2522. https://doi.org/10.3390/nano11102522