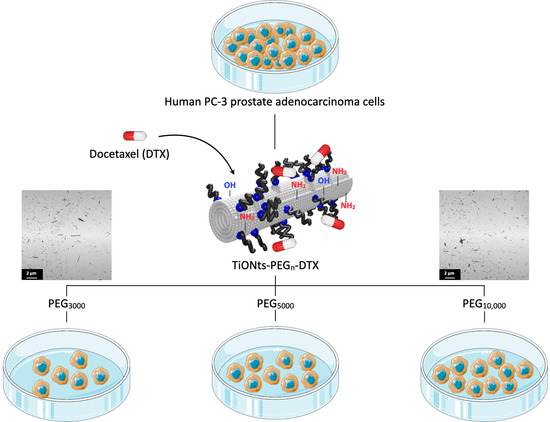
About the Influence of PEG Spacers on the Cytotoxicity of Titanate Nanotubes-Docetaxel Nanohybrids against a Prostate Cancer Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Titanate Nanotube (TiONts) Synthesis
2.3. Amine-Functionalized TiONts (TiONts-APTES) Preparation
2.4. Functionalization of TiONts-APTES by Polyethylene Glycol with Different Ethylene Oxide Chain Lengths (PEG3000/5000/10,000)
2.5. Modification and Grafting of Docetaxel on TiONts-PEGn
2.6. Surface Area Measurements
2.7. Thermogravimetric Analysis (TGA)
2.8. ζ-Potential Measurements
2.9. UV-Visible Absorbance Measurements
2.10. X-ray Photoelectron Spectroscopy (XPS)
2.11. Transmission Electron Microscopy (TEM)
2.12. Fourier Transformed Infrared (FTIR) Spectroscopy
2.13. Inductively Coupled Plasma (ICP) Spectroscopy
2.14. Cell Culture of Human PC-3 Prostate Adenocarcinoma
2.15. In Vitro Evaluation of Nanohybrid Cytotoxicity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Walsh, F.C. Titanate and Titania Nanotubes: Synthesis, Properties and Applications; Royal Society of Chemistry: Cambridge, UK, 2010; p. 154. [Google Scholar]
- Niu, L.; Shao, M.; Wang, S.; Lu, L.; Gao, H.; Wang, J. Titanate nanotubes: Preparation, characterization, and application in the detection of dopamine. J. Mater. Sci. 2008, 43, 1510–1514. [Google Scholar] [CrossRef]
- Papa, A.L.; Dumont, L.; Vandroux, D.; Millot, N. Titanate nanotubes: Towards a novel and safer nanovector for cardiomyocytes. Nanotoxicology 2013, 7, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Cai, Y. Preparation of amino-modified titanate nanotubes and its striking adsorption ability to duplex DNA. J. Nanopart. Res. 2011, 13, 39–43. [Google Scholar] [CrossRef]
- Oh, S.-H.; Finõnes, R.R.; Daraio, C.; Chen, L.-H.; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.-L.; Maurizi, L.; Vandroux, D.; Walker, P.; Millot, N. Synthesis of Titanate Nanotubes Directly Coated with USPIO in Hydrothermal Conditions: A New Detectable Nanocarrier. J. Phys. Chem. C 2011, 115, 19012–19017. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Oudot, A.; Moreau, M.; Boidot, R.; Chassagnon, R.; Saïd, N.M.; Roux, S.; Mirjolet, C.; Millot, N. Titanate Nanotubes Engineered with Gold Nanoparticles and Docetaxel to Enhance Radiotherapy on Xenografted Prostate Tumors. Cancers 2019, 11, 1962. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.; Bernhard, Y.; Boudon, J.; Heintz, O.; Millot, N.; Decreau, R.A. Phthalocyanine-titanate nanotubes: A promising nanocarrier detectable by optical imaging in the so-called imaging window. RSC Adv. 2015, 5, 6315–6322. [Google Scholar] [CrossRef]
- Sallem, F.; Boudon, J.; HEINTZ, O.; Séverin, I.; Megriche, A.; Millot, N. Synthesis and characterization of chitosan-coated titanate nanotubes: Towards a new safe nanocarrier. Dalton Trans. 2017, 46, 15386–15398. [Google Scholar] [CrossRef]
- Sruthi, S.; Loiseau, A.; Boudon, J.; Sallem, F.; Maurizi, L.; Mohanan, P.V.; Lizard, G.; Millot, N. In vitro interaction and biocompatibility of titanate nanotubes with microglial cells. Toxicol. Appl. Pharmacol. 2018, 353, 74–86. [Google Scholar] [CrossRef]
- Baati, T.; Kefi, B.B.; Aouane, A.; Njim, L.; Chaspoul, F.; Heresanu, V.; Kerkeni, A.; Neffati, F.; Hammami, M. Biocompatible titanate nanotubes with high loading capacity of genistein: Cytotoxicity study and anti-migratory effect on U87-MG cancer cell lines. RSC Adv. 2016, 6, 101688–101696. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Mirjolet, C.; Crehange, G.; Millot, N. Taxane-Grafted Metal-Oxide Nanoparticles as a New Theranostic Tool against Cancer: The Promising Example of Docetaxel-Functionalized Titanate Nanotubes on Prostate Tumors. Adv. Healthc. Mater. 2017, 6, 1700245. [Google Scholar] [CrossRef]
- Mirjolet, C.; Boudon, J.; Loiseau, A.; Chevrier, S.; Boidot, R.; Oudot, A.; Collin, B.; Martin, E.; Joy, P.A.; Millot, N. Docetaxel-titanate nanotubes enhance radiosensitivity in an androgen-independent prostate cancer model. Int. J. Nanomed. 2017, 12, 6357. [Google Scholar] [CrossRef] [Green Version]
- Mirjolet, C.; Papa, A.-L.; Créhange, G.; Raguin, O.; Seignez, C.; Paul, C.; Truc, G.; Maingon, P.; Millot, N. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: A proof-of-concept. Radiother. Oncol. 2013, 108, 136–142. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Yoo, J.-W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 3270–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, M.D.; Jay, M.; Dziubla, T.D.; Lu, X. PEGylation of nanocarrier drug delivery systems: State of the art. J. Biomed. Nanotechnol. 2008, 4, 133–148. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.-L.; Boudon, J.; Bellat, V.; Loiseau, A.; Bisht, H.; Sallem, F.; Chassagnon, R.; Berard, V.; Millot, N. Dispersion of titanate nanotubes for nanomedicine: Comparison of PEI and PEG nanohybrids. Dalton Trans. 2015, 44, 739–746. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Sakura, T.; Takahashi, T.; Kataoka, K.; Nagasaki, Y. One-pot preparation of mono-dispersed and physiologically stabilized gold colloid. Colloid Polym. Sci. 2005, 284, 97–101. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Stealth Nanoparticles: High Density but Sheddable PEG is a Key for Tumor Targeting. J. Control Release 2010, 145, 178–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nano 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Hwu, J.R.; Lin, Y.S.; Josephrajan, T.; Hsu, M.-H.; Cheng, F.-Y.; Yeh, C.-S.; Su, W.-C.; Shieh, D.-B. Targeted Paclitaxel by Conjugation to Iron Oxide and Gold Nanoparticles. J. Am. Chem. Soc. 2008, 131, 66–68. [Google Scholar] [CrossRef]
- Dos Santos, N.; Allen, C.; Doppen, A.-M.; Anantha, M.; Cox, K.A.K.; Gallagher, R.C.; Karlsson, G.; Edwards, K.; Kenner, G.; Samuels, L.; et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.-H. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J. Control. Release 2007, 118, 7–17. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Du, X.-J.; Yang, J.-X.; Shen, S.; Li, H.-J.; Luo, Y.-L.; Iqbal, S.; Xu, C.-F.; Ye, X.-D.; Cao, J.; et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials 2018, 182, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.C.; Dufort, S.; Josserand, V.; Perriat, P.; Coll, J.L.; Roux, S.; Tillement, O. Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings. Small 2009, 5, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, B. Polyethylene glycol density and length affects nanoparticle uptake by cancer cells. J. Nanomed. Res. 2014, 1, 00006. [Google Scholar]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; De Rose, R.; Alt, K.; Alcantara, S.; Paterson, B.M.; Liang, K.; Hu, M.; Richardson, J.J.; Yan, Y.; Jeffery, C.M. Engineering poly (ethylene glycol) particles for improved biodistribution. ACS Nano 2015, 9, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Zhong, Y.; Dong, J.; Qian, C.; Sun, S.; Gao, L.; Yang, D. The effect of PEG functionalization on the in vivo behavior and toxicity of CdTe quantum dots. RSC Adv. 2019, 9, 12218–12225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [Green Version]
- Bottini, M.; Rosato, N.; Bottini, N. PEG-modified carbon nanotubes in biomedicine: Current status and challenges ahead. Biomacromolecules 2011, 12, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Morgat, J.-L.; Vert, M.; Mysiakine, E.; Gref, R.; Devissaguet, J.-P.; Barratt, G. Biodistribution of Long-Circulating PEG-Grafted Nanocapsules in Mice: Effects of PEG Chain Length and Density. Pharm. Res. 2001, 18, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, M.; Eric, K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997, 48, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Liu, H.; Kim, S.; Guo, M.; Navarro, V.; Bander, N.H. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: Implications for PSA surrogacy. Prostate 2009, 69, 1579–1585. [Google Scholar] [CrossRef]
- Galsky, M.D.; Vogelzang, N.J. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Liu, S.; Pinski, J. Luteinizing hormone-releasing hormone receptor targeted agents for prostate cancer. Expert Opin. Investig. Drugs 2011, 20, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Pontón, P.I.; d’Almeida, J.R.M.; Marinkovic, B.A.; Savić, S.M.; Mancic, L.; Rey, N.A.; Morgado, E.; Rizzo, F.C. The effects of the chemical composition of titanate nanotubes and solvent type on 3-aminopropyltriethoxysilane grafting efficiency. Appl. Surf. Sci. 2014, 301, 315–322. [Google Scholar] [CrossRef]
- Papa, A.-L.; Millot, N.; Saviot, L.; Chassagnon, R.; Heintz, O. Effect of Reaction Parameters on Composition and Morphology of Titanate Nanomaterials. J. Phys. Chem. C 2009, 113, 12682–12689. [Google Scholar] [CrossRef]
- Peracchia, M.T.; Vauthier, C.; Passirani, C.; Couvreur, P.; Labarre, D. Complement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticles. Life Sci. 1997, 61, 749–761. [Google Scholar] [CrossRef]
- Fang, C.; Shi, B.; Pei, Y.-Y.; Hong, M.-H.; Wu, J.; Chen, H.-Z. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Fee, C.J.; Ruckh, T.; Popat, K.C. Conformational Studies of Covalently Grafted Poly(ethylene glycol) on Modified Solid Matrices Using X-ray Photoelectron Spectroscopy. Langmuir 2010, 26, 7299–7306. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Reuter, K.G.; Kai, M.P.; Herlihy, K.P.; Jones, S.W.; Luft, J.C.; Napier, M.; Bear, J.E.; DeSimone, J.M. PEGylated PRINT nanoparticles: The impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012, 12, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- Movileanu, L.; Cheley, S.; Bayley, H. Partitioning of Individual Flexible Polymers into a Nanoscopic Protein Pore. Biophys. J. 2003, 85, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Horgan, A.; Levicky, R. Reaction of N-phenyl maleimide with aminosilane monolayers. Colloids Surf. B Biointerfaces 2004, 35, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Matov, A.; Beltran, H.; Fontugne, J.; Mosquera, J.M.; Cheung, C.; MacDonald, T.Y.; Sung, M.; O’Toole, S.; Kench, J.G. ERG induces taxane resistance in castration-resistant prostate cancer. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Cao, S.; Yang, Z.; Zhang, S.; Zhang, Q.; Jiang, X. Preparation, characterization and anti-glioma effects of docetaxel-incorporated albumin-lipid nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 2137–2147. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, T.H.; Kim, B.D.; Lee, T.H.; Yoon, H.Y.; Goo, Y.T.; Choi, Y.S.; Kang, M.J.; Choi, Y.W. Enhanced Docetaxel Delivery Using Sterically Stabilized RIPL Peptide-Conjugated Nanostructured Lipid Carriers: In Vitro and In Vivo Antitumor Efficacy Against SKOV3 Ovarian Cancer Cells. Int. J. Pharm. 2020, 119393. [Google Scholar] [CrossRef]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, C.; Motamedchaboki, K.; Magrini, A.; Palmieri, G.; Mattei, M.; Bernardini, S.; Rosato, N.; Bottini, N.; Bottini, M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: Potential implications on biological performance. ACS Nano 2013, 7, 1974–1989. [Google Scholar] [CrossRef]








| Sample | Initial Temperature of Degradation (°C) | Relative Mass Loss (%) | Degraded Molecular Weight (g∙mol−1) | Molecule∙nm−2 (Average) | Number of Loaded Molecules Per TiONt (*) |
|---|---|---|---|---|---|
| TiONts | 190 | 2.6 | 18 | 10.2 (±1.5) OH | - |
| TiONts-APTES | 175 | 6.3 | 58 | 2.6 (±0.2) NH2 | 14,230 |
| TiONts-PEG3000 | 170 | 17.6 | 3073 | 0.090 (±0.005) PEG3000 | 490 |
| TiONts-PEG5000 | 170 | 16.3 | 4847 | 0.050 (±0.003) PEG5000 | 270 |
| TiONts-PEG10,000 | 170 | 18.8 | 9515 | 0.030 (±0.002) PEG10,000 | 160 |
| TiONts-PEG3000-DTX | 150 | 29.4 | 1049 | 0.32 (±0.02) DTX-PMPI | 1750 |
| TiONts-PEG5000-DTX | 150 | 25.3 | 1049 | 0.24 (± 0.02) DTX-PMPI | 1310 |
| TiONts-PEG10,000-DTX | 150 | 19.9 | 1049 | 0.030 (±0.002) DTX-PMPI | 160 |
| Atomic Concentration (%) | C1s | O1s | NaKLL | Ti2p | N1s | Si2p | S2p |
|---|---|---|---|---|---|---|---|
| TiONts | 7.3 | 58.7 | 13.5 | 20.5 | - | - | - |
| Elements (TiONts)/Ti | 0.3 | 2.9 | 0.7 | 1.0 | - | - | - |
| TiONts-APTES | 11.2 | 56.8 | 5.7 | 21.5 | 2.3 | 2.5 | - |
| Elements (TiONts-APTES)/Ti | 0.5 | 2.6 | 0.3 | 1.0 | 0.1 | 0.1 | - |
| TiONts-PEG3000 | 24.1 | 55.5 | - | 15.9 | 2.3 | 1.9 | 0.3 |
| Elements (TiONts-PEG3000)/Ti | 1.5 | 3.5 | - | 1.0 | 0.2 | 0.1 | 0.02 |
| TiONts-PEG5000 | 25.1 | 55.2 | - | 15.2 | 2.2 | 2.1 | 0.2 |
| Elements (TiONts-PEG5000)/Ti | 1.6 | 3.6 | - | 1.0 | 0.2 | 0.2 | 0.01 |
| TiONts-PEG10,000 | 26.0 | 55.7 | - | 14.5 | 2.3 | 1.5 | - |
| Elements (TiONts-PEG10,000)/Ti | 1.8 | 3.8 | - | 1.0 | 0.2 | 0.1 | - |
| Sample | Control | Nanohybrid Name and Its Corresponding Docetaxel Concentration | |||
|---|---|---|---|---|---|
| TiONts-PEG3000-DTX | TiONts-PEG10,000-DTX | ||||
| 10 nM | 20 nM | 10 nM | 20 nM | ||
| Nanohybrid Mass Per Cells (µg/106 Cells) | 1.1 | 64 | 335 | 72 | 179 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiseau, A.; Boudon, J.; Mirjolet, C.; Morgand, V.; Millot, N. About the Influence of PEG Spacers on the Cytotoxicity of Titanate Nanotubes-Docetaxel Nanohybrids against a Prostate Cancer Cell Line. Nanomaterials 2021, 11, 2733. https://doi.org/10.3390/nano11102733
Loiseau A, Boudon J, Mirjolet C, Morgand V, Millot N. About the Influence of PEG Spacers on the Cytotoxicity of Titanate Nanotubes-Docetaxel Nanohybrids against a Prostate Cancer Cell Line. Nanomaterials. 2021; 11(10):2733. https://doi.org/10.3390/nano11102733
Chicago/Turabian StyleLoiseau, Alexis, Julien Boudon, Céline Mirjolet, Véronique Morgand, and Nadine Millot. 2021. "About the Influence of PEG Spacers on the Cytotoxicity of Titanate Nanotubes-Docetaxel Nanohybrids against a Prostate Cancer Cell Line" Nanomaterials 11, no. 10: 2733. https://doi.org/10.3390/nano11102733
APA StyleLoiseau, A., Boudon, J., Mirjolet, C., Morgand, V., & Millot, N. (2021). About the Influence of PEG Spacers on the Cytotoxicity of Titanate Nanotubes-Docetaxel Nanohybrids against a Prostate Cancer Cell Line. Nanomaterials, 11(10), 2733. https://doi.org/10.3390/nano11102733