Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion
Abstract
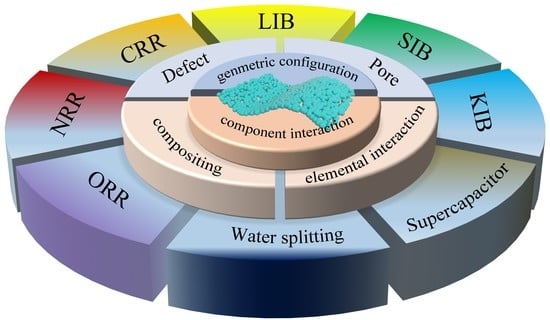
:1. Introduction
2. Manipulation Strategy of 2D ANMs
2.1. Synthesis Methods
2.2. Manipulation Modes
2.2.1. Geometric Configuration Design
Spatial Structure Design
Coordination Environment Design
2.2.2. Component Interaction
Elemental Interaction
Heterophase Compositing
3. Manipulating 2D ANMs for Batteries and Supercapacitors
3.1. Rechargeable Battery
3.2. Supercapacitor
4. Manipulating 2D ANMs for Electrocatalysis
4.1. Water Splitting
4.2. Electrochemical Reduction Reactions
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simonbc, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 17059. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, Z.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Y.; Zheng, S.; Guo, X.; Xue, H.; Pang, H. Recent progress in some amorphous materials for supercapacitors. Small 2018, 14, 1800426–1800444. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, Y.; Wang, C.; Li, Q.; Xiang, J.; Liang, L.; Wu, M.; Zhao, H.; Lei, Y. Amorphous TiO2 inverse opal anode for high-rate sodium ion batteries. Nano Energy 2017, 31, 514–524. [Google Scholar] [CrossRef]
- Toh, C.; Zhang, H.; Lin, J.; Mayorov, A.S.; Wang, Y.-P.; Orofeo, C.M.; Ferry, D.B.; Andersen, H.; Kakenov, N.; Guo, Z.; et al. Synthesis and properties of free-standing monolayer amorphous carbon. Nature 2020, 557, 199–203. [Google Scholar] [CrossRef]
- Bellus, M.Z.; Yang, Z.; Hao, J.; Lau, S.P.; Zhao, H. Amorphous two-dimensional black phosphorus with exceptional photocarrier transport properties. 2D Mater. 2017, 4, 025063. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.F.; Li, Y.H.; Wang, C.W.; Zu, M.Y.; Fu, H.Q.; Yang, X.H.; Yang, H.G. Accelerating neutral hydrogen evolution with tungsten modulated amorphous metal hydroxides. ACS Catal. 2018, 8, 5200–5205. [Google Scholar] [CrossRef]
- Radhakrishnan, T.; Aparna, M.P.; Chatanathodi, R.; Sandhyarani, N. Amorphous rhenium disulfide nanosheets: A methanol-tolerant transition metal dichalcogenide catalyst for oxygen reduction reaction. ACS Appl. Nano Mater. 2019, 2, 4480–4488. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wang, C.; Yang, G. A simple electrochemical route to access amorphous mixed-metal hydroxides for supercapacitor electrode materials. Adv. Energy Mater. 2014, 5, 1401767. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Dubal, D.P.; Ji, S.-H.; Kim, D.-H. Self-assembled nickel pyrophosphate-decorated amorphous bimetal hydroxides 2D-on-2D nanostructure for high-energy solid-state asymmetric supercapacitor. Small 2019, 15, 1901145. [Google Scholar] [CrossRef]
- Liu, J.; Ji, Y.; Nai, J.; Niu, X.; Luo, Y.; Guo, L.; Yang, S. Ultrathin amorphous cobalt-vanadium hydr (oxy) oxide catalysts for the oxygen evolution reaction. Energy Environ. Sci. 2018, 11, 1736–1741. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Si, W.; Wu, H. Mass production of two-dimensional oxides by rapid heating of hydrous chlorides. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Fei, B.; Cai, G.; Ha, Y.; Liu, J.; Jia, H.; Zhang, J.; Liu, M.; Wu, R. Boronization-induced ultrathin 2D nanosheets with abundant crystalline-amorphous phase boundary supported on nickel foam toward efficient water splitting. Adv. Energy Mater. 2020, 10, 1902714. [Google Scholar] [CrossRef]
- Yuan, T.; Hu, Z.; Zhao, Y.; Fang, J.; Lv, J.; Zhang, Q.; Zhuang, Z.; Gu, L.; Hu, S. Two-dimensional amorphous SnOx from liquid metal: Mass production, phase transfer, and electrocatalytic CO2 reduction toward formic acid. Nano Lett. 2020, 20, 2916–2922. [Google Scholar] [CrossRef]
- Kotakoski, J.; Krasheninnikov, A.V.; Kaiser, U.; Meyer, J.C. From point defects in graphene to two-dimensional amorphous carbon. Phys. Rev. Lett. 2011, 106, 105505. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Zhao, H.; Wong, K.; Lei, D.; Zhang, Y.; Quan, X. Covalent functionalization of MoS2 nanosheets synthesized by liquid phase exfoliation to construct electrochemical sensors for Cd (II). Talanta 2017, 182, 38–48. [Google Scholar] [CrossRef]
- Fu, W.; Yang, S.; Yang, H.; Guo, B.; Huang, Z. 2D amorphous MoS3 nanosheets with porous network structures for scavenging toxic metal ions from synthetic acid mine drainage. J. Mater. Chem. A 2019, 7, 18799–18806. [Google Scholar] [CrossRef]
- Selvaraj, A.R.; Muthusamy, A.; Inho-Cho; Kim, H.-J.; Senthil, K.; Prabakara, K. Ultrahigh surface area biomass derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Dang, L.; Zhang, H.; Wu, X.; Yang, B.; Li, Z.; Zhang, X.; Lei, L.; Jin, S. Amorphous cobalt-iron hydroxide nanosheet electrocatalyst for efficient electrochemical and photo-electrochemical oxygen evolution. Adv. Funct. Mater. 2017, 27, 1603904. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Mishra, I.K.; Luo, D.; Yu, F.; Yu, Y.; Chen, S.; Ren, Z. Amorphous NiFe layered double hydroxide nanosheets decorated on 3D nickel phosphide nanoarrays: A hierarchical core-shell electrocatalyst for efficient oxygen evolution. J. Mater. Chem. A 2018, 6, 13619–13623. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.; Li, F.; Hao, R.; Wang, S.; Guo, L. A generalized strategy for the synthesis of large-size ultrathin two-dimensional metal oxide nanosheets. Angew. Chem. Int. Ed. 2017, 129, 8892–8896. [Google Scholar] [CrossRef]
- Jia, B.; Hao, R.; Huang, Z.; Hu, P.; Li, L.; Zhang, Y.; Guo, L. Creating ultrathin amorphous metal hydroxide and oxide nanosheet libraries. J. Mater. Chem. A 2019, 7, 4383–4388. [Google Scholar] [CrossRef]
- Zhao, H.; Yue, Y.; Zhang, Y.; Li, L.; Guo, L. Ternary artificial nacre reinforced by ultrathin amorphous alumina with exceptional mechanical properties. Adv. Mater. 2016, 28, 2037–2042. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, Y.; Pan, Z.; Meng, Y.; Jiang, L.; Wu, Z.; Yang, P.; Gu, Q.; Sun, D.; Hu, L. Rapid amorphization in metastable CoSeO3·H2O nanosheets for ultrafast lithiation kinetics. ACS Nano 2018, 12, 5011–5020. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Cui, W.; Zhu, C.; Qi, Y. CO2-assisted fabrication of two-dimensional amorphous molybdenum oxide nanosheets for enhanced plasmon resonances. Angew. Chem. Int. Ed. 2017, 56, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Du, C.; Yan, B.; Wang, C.; Yang, G. Two-dimensional amorphous NiO as a plasmonic photocatalyst for solar H2 evolution. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wan, X.; Qu, Y.; Chen, W.; Lin, Y.; Li, H.; Han, X.; et al. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, C.; Fan, A.; Ren, D.; Luan, C.; Yang, J.; Liu, Y.; Zhang, X.; Dai, X.; Wang, M. Amorphous NiMS (M: Co, Fe or Mn) holey nanosheets derived from crystal phase transition for enhanced oxygen evolution in water splitting. Electrochim. Acta 2019, 323, 134756. [Google Scholar] [CrossRef]
- Jia, B.; Yang, J.; Hao, R.; Li, L.; Guo, L. Confined synthesis of ultrathin amorphous metal-oxide nanosheets. ACS Mater. Lett. 2020, 2, 610–615. [Google Scholar] [CrossRef]
- Thomas, S.; Jung, H.; Kim, S.; Jun, B.; Lee, C.; Lee, S. Two-dimensional haeckelite h567: A promising high capacity and fast Li diffusion anode material for lithium-ion batteries. Carbon 2019, 148, 344–353. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Du, N.; Hou, W. Amorphous molybdenum sulfide monolayer nanosheets for highly efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2020, 398, 125685. [Google Scholar] [CrossRef]
- Wei, Q.; Tan, X.; Zhang, J.; Yang, L.; Cao, L.; Dong, B. Fe doped amorphous single layered vanadyl phosphate nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interface Sci. 2021, 586, 505–513. [Google Scholar] [CrossRef]
- Zeng, L.; Cao, B.; Wang, X.; Liu, H.; Shang, J.; Lang, J.; Cao, X.; Gu, H. Ultrathin amorphous iron-doped cobalt-molybdenum hydroxide nanosheets for advanced oxygen evolution reactions. Nanoscale 2021, 13, 3153–3160. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Liu, D.; Tan, H.; Dinh, K.N.; Yang, L.; Ren, H.; Huang, W.; Fang, W.; Yao, J.; et al. Amorphous/crystalline heterostructured cobalt-vanadium-iron (oxy) hydroxides for highly efficient oxygen evolution reaction. Adv. Energy Mater. 2020, 10, 2002215. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Ning, P.; Chen, X.; Liang, J.; Yao, X.; Chen, D.; Qin, L.; Huang, Y.; Wen, Z. 2D heterostructure of amorphous CoFeB coating black phosphorus nanosheets with optimal oxygen intermediate absorption for improved electrocatalytic water oxidation. ACS Nano 2021, 15, 12418–12428. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Yun, J.-Y.; Lim, J.-H.; Yoo, B. Enhanced electrocatalytic properties of electrodeposited amorphous cobalt-nickel hydroxide nanosheets on nickel foam by the formation of nickel nanocones for the oxygen evolution reaction. J. Alloys Compd. 2017, 693, 964–969. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Zhang, N.; Liu, X.-X. Amorphous NiFe(oxy)hydroxide nanosheet integrated partially exfoliated graphite foil for high efficiency oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 24208–24216. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Li, H.B.; Yang, G.W. Amorphous Co (OH)2 nanosheet electrocatalyst and the physical mechanism for its high activity and long-term cycle stability. J. Appl. Phys. 2016, 119, 034902. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Shi, X.; Asiri, A.M.; Wang, L.; Li, X.; Sun, X.; Zhang, Q.; Wang, H. A platinum oxide decorated amorphous cobalt oxide hydroxide nanosheet array towards alkaline hydrogen evolution. J. Mater. Chem. A 2018, 6, 3864–3868. [Google Scholar] [CrossRef]
- Mahmood, N.; Tang, T.; Hou, Y. Nanostructured anode materials for lithium ion batteries: Progress, challenge and perspective. Adv. Energy Mater. 2016, 6, 1600374. [Google Scholar] [CrossRef]
- Jia, B.; Chen, W.; Luo, J.; Yang, Z.; Li, L.; Guo, L. Construction of MnO2 artificial leaf with atomic thickness as highly stable battery anodes. Adv. Mater. 2019, 32, 1906582. [Google Scholar] [CrossRef]
- Yan, P.; Ji, L.; Liu, X.; Guan, Q.; Guo, J.; Shen, Y.; Zhang, H.; Wei, W.; Cui, X.; Xu, Q. 2D amorphous-MoO3-x@Ti3C2-MXene non-van der Waals heterostructures as anode materials for lithium-ion batteries. Nano Energy 2021, 86, 106139. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Huang, S.; Liu, L.; Wang, Y.; Jin, J.; Kong, D.; Zhang, L.; Schmidtbe, O.G. PVD customized 2D porous amorphous silicon nanoflakes percolated with carbon nanotubes for high areal capacity lithium ion batteries. J. Mater. Chem. A 2020, 8, 4836–4843. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ma, S.; Tong, S.; Han, X.; Wang, H. Flexible amorphous MoS2 nanoflakes/N-doped carbon microtubes/reduced graphite oxide composite paper as binder free anode for full cell lithium ion batteries. Electrochim. Acta 2020, 333, 135568. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, J.; Hua, W.; Liu, Y.; Wang, J.; Liang, Y.; Lai, W.; Jiang, Y.; Huang, Y.; Zhang, W.; et al. Architecting amorphous vanadium oxide/MXene nanohybrid via tunable anodic oxidation for high- performance sodium-ion batteries. Adv. Energy Mater. 2021, 11, 2100757. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, S.; Wang, L.; Yao, Y.; Shao, R.; Shen, L.; Yu, L.; Dai, J.; Jiang, Y.; Cheng, X.; et al. Harnessing the Volume Expansion of MoS3 Anode by Structure Engineering to Achieve High Performance Beyond Lithium-Based Rechargeable Batteries. Adv. Mater. 2021, 33, 2106232. [Google Scholar] [CrossRef]
- Hou, H.; Shao, L.; Zhang, Y.; Zou, G.; Chen, J.; Ji, X. Large-area carbon nanosheets doped with phosphorus: A high-performance anode material for sodium-ion batteries. Adv. Sci. 2017, 4, 1600243. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Gao, J.; Wu, G.; Liu, P.; Guo, W.; Zhou, H.; Ge, J.; Hu, Y.; Xue, Z.; Li, H.; et al. Amorphous metal oxide nanosheets featuring reversible structure transformations as sodium-ion battery anodes. Cell Rep. 2020, 1, 100118. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, Y.; Xu, J.; Ma, J.; Zhou, X.; Bao, J. Construction of amorphous FePO4 nanosheets with enhanced sodium storage properties. ACS Appl. Energy Mater. 2018, 1, 4395–4402. [Google Scholar] [CrossRef]
- Fa, X.; Yu, C.; Yang, J.; Ling, Z.; Hu, C.; Zhang, M.; Qiu, J. A Layered-nanospace-confinement strategy for the synthesis of two-dimensional porous carbon nanosheets for high-rate performance supercapacitors. Adv. Energy Mater. 2015, 5, 1401761. [Google Scholar]
- Huang, C.; Song, X.; Qin, Y.; Xu, B.; Chen, H.C. Cation exchange reaction derived amorphous bimetal hydroxides as advanced battery materials for hybrid supercapacitors. J. Mater. Chem. A 2018, 6, 21047–21055. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, N.; He, Y.; Liang, B.; Ma, R.; Liu, X. Controllable fabrication of amorphous Co-Ni pyrophosphates for tuning electrochemical performance in supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 23114–23121. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Cao, H.; Song, X.; Huang, C.; Feng, H.; Zhao, X.S. Synthesis of amorphous nickel-cobalt-manganese hydroxides for supercapacitor-battery hybrid energy storage system. Energy Storage Mater. 2019, 17, 194–203. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, W.; Zhu, S.; Liang, Y.; Cui, Z.; Yang, X.; Inoue, A. Low-cost fabrication of amorphous cobalt-iron-boron nanosheets for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 296, 198–205. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Wang, Y.; Yang, Z.; Fan, X.; Liu, L.-M.; Guo, L. Achieving delafossite analog by in situ electrochemical self-reconstruction as an oxygen-evolving catalyst. Proc. Natl. Acad. Sci. USA 2020, 117, 21906–21913. [Google Scholar] [CrossRef]
- Haq, T.u.; Mansour, S.A.; Munir, A.; Haik, Y. Gold-supported gadolinium doped CoB amorphous sheet: A new benchmark electrocatalyst for water oxidation with high turnover frequency. Adv. Funct. Mater. 2020, 30, 1910309. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Huang, H.; Han, X.; Liu, Z.; Qiu, J. Co ion-intercalation amorphous and ultrathin microstructure for high-rate oxygen evolution. Energy Storage Mater. 2018, 10, 291–296. [Google Scholar] [CrossRef]
- Ren, X.; Wu, D.; Ge, R.; Sun, X.; Ma, H.; Yan, T.; Zhang, Y.; Du, B.; Wei, Q.; Chen, L. Self-supported CoMoS4 nanosheet array as an efficient catalyst for hydrogen evolution reaction at neutral pH. Nano Res. 2018, 11, 2024–2033. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jiang, H.; Hu, Y.; Saha, P.; Li, C. Mo-triggered amorphous Ni3S2 nanosheets as efficient and durable electrocatalysts for water splitting. Mater. Chem. Front. 2018, 2, 1462–1466. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Teng, W.; Shi, P. Amorphous Nickel-Cobalt-Borate nanosheet arrays for efficient and durable water oxidation electrocatalysis under near-neutral conditions. Chem. Eur. J. 2017, 23, 9741–9745. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhou, L.; Zheng, Q.; Xie, F.; Lam, K.H.; Lin, D. Ultrathin amorphous CoFeP nanosheets derived from CoFe LDHs by partial phosphating as excellent bifunctional catalysts for overall water splitting. Electrochim. Acta 2019, 323, 134595. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Q.; Luo, J.; Fu, H.C.; Chen, X.H.; Wu, L.L.; Luo, H.Q.; Li, N.B. Heteroatoms adjusting amorphous FeMn-based nanosheets via a facile electrodeposition method for full water splitting. ACS Sustain. Chem. Eng. 2021, 9, 5963–5971. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Yan, Z.; Chen, X.; Jiao, L.; Cheng, F.; Chen, J. Superhydrophilic amorphous Co-B-P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 22062–22069. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-rich RuCu nanosheets for pH-Universal overall water splitting electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, J.; Da, Y.; Zhang, J.; Li, L.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Sequential electrodeposition of bifunctional catalytically active structures in MoO3/Ni-NiO composite electrocatalysts for selective hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 2003414. [Google Scholar] [CrossRef]
- Cao, D.; Wang, J.; Xu, H.; Cheng, D. Growth of highly active amorphous RuCu nanosheets on Cu nanotubes for the hydrogen evolution reaction in wide pH values. Small 2020, 16, 2000924. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Dai, X.; Qiao, H.; Yong, J.; Luan, X.; Yu, L.; Luan, C.; Wang, Y.; Zhang, X. NiCo-DH nanodots anchored on amorphous NiCo-Sulfide sheets as efficient electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2019, 295, 1085–1092. [Google Scholar] [CrossRef]
- Sukanya, R.; Chen, S.-M. Amorphous cobalt boride nanosheets anchored surface-functionalized carbon nanofiber: An bifunctional and efficient catalyst for electrochemical sensing and oxygen evolution reaction. J. Colloid Interface Sci. 2020, 580, 318–331. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, F.; Wu, F.; Lu, H.; Li, L. Ultrathin Amorphous Ni(OH)2 Nanosheets on Ultrathin α-Fe2O3 Films for Improved Photoelectrochemical Water Oxidation. Adv. Mater. Interfaces 2016, 3, 1600256. [Google Scholar] [CrossRef]
- Fang, M.; Han, D.; Xu, W.-B.; Shen, Y.; Lu, Y.; Cao, P.; Han, S.; Xu, W.; Zhu, D.; Liu, W.; et al. Surface-guided formation of amorphous mixed-metal oxyhydroxides on ultrathin MnO2 nanosheet arrays for efficient electrocatalytic oxygen evolution. Adv. Energy Mater. 2020, 10, 2001059. [Google Scholar] [CrossRef]
- Niu, Y.; Li, W.; Wu, X.; Feng, B.; Yu, Y.; Hu, W.; Li, C.M. Amorphous nickel sulfide nanosheets with embedded vanadium oxide nanocrystals on nickel foam for efficient electrochemical water oxidation. J. Mater. Chem. A 2019, 7, 10534–10542. [Google Scholar] [CrossRef]
- Masa, J.; Sinev, I.; Mistry, H.; Ventosa, E.; de la Mata, M.; Arbiol, J.; Muhler, M.; Cuenya, B.R.; Schuhmann, W. Ultrathin high surface area nickel boride (NixB) nanosheets as highly efficient electrocatalyst for oxygen evolution. Adv. Energy Mater. 2017, 7, 1700381. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Liu, Y.; Li, G.-D.; Wu, Y.; Liu, D.-P.; Li, W.; Li, H.-W.; Wang, D.; Zhang, Y.; Zou, X. Ultrafast formation of amorphous bimetallic hydroxide films on 3D conductive sulfide nanoarrays for large-current-density oxygen evolution electrocatalysis. Adv. Mater. 2017, 29, 1700404. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yu, B.-B.; Yin, X.-L.; Jiang, W.-J.; Jiang, Y.; Hu, J.-S.; Wan, L.-J. Physical vapor deposition of amorphous MoS2 nanosheet arrays on carbon cloth for highly reproducible large-area electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 19277–19281. [Google Scholar] [CrossRef]
- Ahn, B.-W.; Kim, T.-Y.; Kim, S.-H.; Song, Y.-I.; Suh, S.-J. Amorphous MoS2 nanosheets grown on copper@nickel-phosphorous dendritic structures for hydrogen evolution reaction. Appl. Surf. Sci. 2018, 432, 183–189. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Liu, D.; Yu, Y.; Zhang, B. Enhancing oxygen evolution reaction at high current densities on amorphous-like Ni-Fe-S ultrathin nanosheets via oxygen incorporation and electrochemical tuning. Adv. Sci. 2017, 4, 1600343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Z.; Meng, H.; Sun, J.; Guo, N.; Xue, H.; Huang, K.; He, F.; Li, F.; Wang, Q. Engineering of amorphous structures and sulfur defects into ultrathin FeS nanosheets to achieve superior electrocatalytic alkaline oxygen evolution. ACS Appl. Mater. Interfaces 2020, 12, 51846–51853. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Cheng, Y.; Zhang, Y.; Pang, H. Amorphous cobalt phosphate porous nanosheets derived from two-dimensionalcobalt phosphonate organic frameworks for high performance of oxygen evolution reaction. Appl. Mater. Today 2020, 18, 100517. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, L.; Chen, R.; Wang, Y.; Xie, C.; Tao, L.; Huang, L.; Wang, S. One-step, room temperature generation of porous and amorphous cobalt hydroxysulfides from layered double hydroxides for superior oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 24311–24316. [Google Scholar] [CrossRef]
- Sial, M.A.Z.G.; Lin, H.; Wang, X. Microporous 2D NiCoFe phosphate nanosheets supported on Ni foam for efficient overall water splitting in alkaline media. Nanoscale 2018, 10, 12975–12980. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Huang, J.; Xi, Y.; Gao, R.; Su, G.; Wang, W.; Cao, L.; Dong, B. Vertical growth of 2D amorphous FePO4 nanosheet on Ni foam: Outer and inner structural design for superior water splitting. Adv. Mater. 2017, 29, 1704574. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Zhang, J.; Yu, C.; Cai, R.; Wang, J.; Zhang, Y.; Wu, J.; Wu, Y. Tuning morphology and electronic structure of amorphous NiFeB nanosheets for enhanced electrocatalytic N2 reduction. ACS Appl. Energy Mater. 2020, 3, 9516–9522. [Google Scholar] [CrossRef]
- Huang, H.; Xia, L.; Cao, R.; Niu, Z.; Chen, H.; Liu, Q.; Li, T.; Shi, X.; Asiri, A.M.; Sun, X. A Biomass-derived carbon-based electrocatalyst for efficient N2 fixation to NH3 under ambient conditions. Chem. Eur. J. 2019, 25, 1914–1917. [Google Scholar] [CrossRef]
- Li, Q.; Kong, D.; Zhao, X.; Cai, Y.; Ma, Z.; Huang, Y.; Wang, H. Short-range amorphous carbon nanosheets for oxygen reduction electrocatalysis. Nanoscale 2020, 2, 5769–5776. [Google Scholar] [CrossRef]
- Poon, K.C.; Wan, W.Y.; Su, H.; Sato, H. One-minute synthesis via electroless reduction of amorphous phosphorus-doped graphene for oxygen reduction reaction. ACS Appl. Energy Mater. 2021, 4, 5388–5391. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Peng, Y.; Fisher, A.; Wang, X. Highly efficient and durable Pd hydride nanocubes embedded in 2D amorphous NiB nanosheets for oxygen reduction reaction. Adv. Energy Mater. 2017, 7, 1700919. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, W.; Ding, L.; Wu, Z.; Gao, F. Au Nanocrystals@defective amorphous MnO2 nanosheets Core/Shell nanostructure with effective CO2 adsorption and activation toward CO2 electroreduction to CO. ACS Sustain. Chem. Eng. 2021, 9, 5230–5239. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Xu, Q.; Yan, P.; Niu, C.; Shen, Y.; Yuan, P.; Jia, Y. Anderson localization in 2D amorphous MoO3-x monolayers for electrochemical ammonia synthesis. ChemCatChem 2019, 11, 5412–5416. [Google Scholar] [CrossRef]
- Chu, K.; Nan, H.; Li, Q.; Guo, Y.; Tian, Y.; Liu, W. Amorphous MoS3 enriched with sulfur vacancies for efficient electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 53, 132–138. [Google Scholar] [CrossRef]
- Chu, K.; Gu, W.; Li, Q.; Liu, Y.; Tian, Y.; Liu, W. Amorphization activated FeB2 porous nanosheets enable efficient electrocatalytic N2 fixation. J. Energy Chem. 2021, 53, 82–89. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hao, R.; Jia, B.; Zhao, H.; Guo, L. Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion. Nanomaterials 2021, 11, 3246. https://doi.org/10.3390/nano11123246
Liu J, Hao R, Jia B, Zhao H, Guo L. Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion. Nanomaterials. 2021; 11(12):3246. https://doi.org/10.3390/nano11123246
Chicago/Turabian StyleLiu, Juzhe, Rui Hao, Binbin Jia, Hewei Zhao, and Lin Guo. 2021. "Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion" Nanomaterials 11, no. 12: 3246. https://doi.org/10.3390/nano11123246
APA StyleLiu, J., Hao, R., Jia, B., Zhao, H., & Guo, L. (2021). Manipulation on Two-Dimensional Amorphous Nanomaterials for Enhanced Electrochemical Energy Storage and Conversion. Nanomaterials, 11(12), 3246. https://doi.org/10.3390/nano11123246