Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study
Abstract
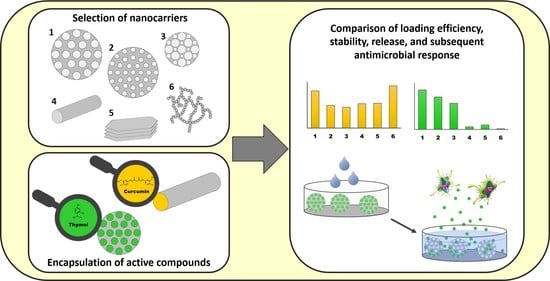
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of the Porous Silica Particles (SPs)
2.3. Nanocarrier Characterization Methods
2.4. Loading of the Active Components to the Nanocarriers
2.5. PEG-Adsorption on the Loaded Nanocarriers
2.6. Determination of the Loaded Amount of Active Components into the Nanocarriers
2.7. Evaluation of the Release and/or Volatilization of Active Components from the Nanocarriers
2.8. Determination of the Antimicrobial Response of the Released Active Compound
3. Results and Discussion
3.1. Design and Characterization of the Nanocarriers for the Active Compounds
3.2. Loading Capacity of the Volatile and the Non-Volatile Model Compounds in the Nanocarriers
3.3. Stability and Release of the Encapsulated Active Compounds
3.4. Antimicrobial Response of the Loaded Nanocarriers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, 135, 3–11. [Google Scholar]
- Khaneghah, A.M.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [Green Version]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.; Kiran, S. Essential oils: A traditionally realized natural resource for food preservation. Curr. Sci. 2016, 110, 1890–1892. [Google Scholar]
- Hassoun, A.; Çoban, Ö.E. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manso, S.; Becerril, R.; Nerín, C.; Gómez-Lus, R. Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- US Food and Drug Administration, Code of Federal Regulations (CFR). Title 21: Food and Drugs, Part 182: Substances Generally Recognized as Safe. Sec. 182.20: Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (Including Distillates). Revised as of 1 April 2019. Available online: https://www.law.cornell.edu/cfr/text/21/182.20 (accessed on 13 January 2021).
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Otero, V.; Becerril, R.; Santos, J.A.; Rodríguez-Calleja, J.M.; Nerín, C.; García-López, M.L. Evaluation of two antimicrobial packaging films against Escherichia coli O157: H7 strains in vitro and during storage of a Spanish ripened sheep cheese (Zamorano). Food Control 2014, 42, 296–302. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; Dos Anjos, R.S.; Riella, H.G.; De Araújo, P.H.H.; De Oliveira, D.; Fiori, M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Wyser, Y.; Adams, M.; Avella, M.; Carlander, D.; Garcia, L.; Pieper, G.; Pieper, G.; Rennen, M.; Schuermans, J.; Weiss, J. Outlook and challenges of nanotechnologies for food packaging. Packag. Technol. Sci. 2016, 29, 615–648. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent developments in food packaging based on nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Lazzara, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite nanotubes for efficient loading, stabilization and controlled release of insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yuan, P.; Annabi-Bergaya, F.; Yu, H.; Liu, D.; Liu, H.; He, H. Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen. Microporous Mesoporous Mater. 2013, 179, 89–98. [Google Scholar] [CrossRef]
- Tas, B.A.; Sehit, E.; Tas, C.E.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Carvacrol loaded halloysite coatings for antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 20, 100300. [Google Scholar]
- Shemesh, R.; A Krepker, M.; Natan, M.; Daninpoleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/halloysite nanotube films with sustained carvacrol release for broad-spectrum antimicrobial activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Krepker, M.; Shemesh, R.; Poleg, Y.D.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar]
- EU Commission. Commission regulation (EU) no 231/2012 of 9 March 2012 laying down specifications for food additives listed in annexes II and III to regulation (EC) no 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2012, 83, 270–271. [Google Scholar]
- US Food and Drug Administration, Code of Federal Regulations (CFR). Title 21: Food and Drugs, Part 172: Food Additives Permitted for Direct Addition to Food for Human Consumption. Sec. 172.480: Silicon Dioxide. Revised as of 1 April 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.480 (accessed on 13 January 2021).
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; M Lagaron, J. Electrospun antimicrobial films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Cheng, M.; Wang, X.; Wang, J. Bioactive mesoporous nano-silica/potato starch films against molds commonly found in post-harvest white mushrooms. Food Hydrocoll. 2019, 95, 517–525. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Pérez-Esteve, É.; Bernardos, A.; Sancenón, F.; Martínez-Máñez, R.; Marcos, M.D.; Barat, J.M. Enhanced antimicrobial activity of essential oil components immobilized on silica particles. Food Chem. 2017, 233, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.M.; Gortzi, O.; Izadi, M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Alvarado, N.; Romero, J.; Torres, A.; de Dicastillo, C.L.; Rojas, A.; Galotto, M.J.; Guarda, A. Supercritical impregnation of thymol in poly (lactic acid) filled with electrospun poly (vinyl alcohol)-cellulose nanocrystals nanofibers: Development an active food packaging material. J. Food Eng. 2018, 217, 1–10. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-density polyethylene/curcumin melt extruded composites with enhanced water vapor barrier and antioxidant properties for active food packaging. Polymer 2019, 175, 137–145. [Google Scholar]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; López-Rubio, A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Baysal, G.; Doğan, F. Investigation and preparation of biodegradable starch-based nanofilms for potential use of curcumin and garlic in food packaging applications. J. Biomater. Sci. Polym. Ed. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Cheng, Y.; Ge, C.; Lodge, J.; Santhanam, K.S.V.; Lu, L. Evaluation of natural plant powders with potential use in antimicrobial packaging applications. J. Appl. Packag. Res. 2014, 6, 4. [Google Scholar]
- European Food Safety Authority. Refined exposure assessment for curcumin (E 100). EFSA J. 2014, 12, 3876. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the re-evaluation of curcumin (E 100) as a food additive. EFSA J. 2010, 8, 1679. [Google Scholar]
- US Food and Drug Administration. GRAS Notice Inventory, GRAS Notice (GRN) No. 822; US Food and Drug Administration: Rockville, MD, USA, 2018.
- US Food and Drug Administration, Code of Federal Regulations (CFR). Title 21: Food and Drugs, Part 172: Food Additives Permitted for Direct Addition to Food for Human Consumption. Sec. 172.515: Synthetic Flavoring Substances and Adjuvants. Revised as of 1 April 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515 (accessed on 13 January 2021).
- Kumar, D.; Schumacher, K.; von Hohenesche, C.D.F.; Grün, M.; Unger, K.K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterisation. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 109–116. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect engineering: Tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Gulin-Sarfraz, T.; Jonasson, S.; Wigenstam, E.; von Haartman, E.; Bucht, A.; Rosenholm, J.M. Feasibility Study of Mesoporous Silica Particles for Pulmonary Drug Delivery: Therapeutic Treatment with Dexamethasone in a Mouse Model of Airway Inflammation. Pharmaceutics 2019, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenholm, J.M.; Lindén, M. Wet-chemical analysis of surface concentration of accessible groups on different amino-functionalized mesoporous SBA-15 silicas. Chem. Mater. 2007, 19, 5023–5034. [Google Scholar] [CrossRef]
- Zhou, C.; Tong, D.; Yu, W. Smectite Nanomaterials: Preparation, Properties, and Functional Applications. In Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–364. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Goletto, V.; Dagry, V.; Babonneau, F. One-pot synthesis of a cubic silicate phase functionalized with phenyl groups. Mrs Online Proc. Libr. Arch. 1999, 576. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Gulin-Sarfraz, T.; Mamaeva, V.; Niemi, R.; Özliseli, E.; Desai, D.; Antfolk, D.; Von Haartman, E.; Lindberg, D.; Prabhakar, N.; et al. Prolonged Dye Release from Mesoporous Silica-Based Imaging Probes Facilitates Long-Term Optical Tracking of Cell Populations In Vivo. Small 2016, 12, 1578–1592. [Google Scholar] [CrossRef]
- Burkett, S.L.; Sims, S.D.; Mann, S. Synthesis of hybrid inorganic–organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem. Commun. 1996, 11, 1367–1368. [Google Scholar] [CrossRef]
- Kim, S.; Diab, R.; Joubert, O.; Canilho, N.; Pasc, A. Core–shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf. B Biointerfaces 2016, 140, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Voon, L.K.; Pang, S.C.; Chin, S.F. Optimizing Delivery Characteristics of Curcumin as a Model Drug via Tailoring Mean Diameter Ranges of Cellulose Beads. Int. J. Polym. Sci. 2017, 2017, 2581767. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.; Zhu, R.; Yu, C.; Wang, S. Rational design of multifunctional dendritic mesoporous silica nanoparticles to load curcumin and enhance efficacy for breast cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 26511–26523. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Da Silva, A.C.; de Freitas Santos, P.D.; do Prado Silva, J.T.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Technol. 2018, 72, 74–82. [Google Scholar] [CrossRef]











| Nano-Carrier | Morphology | Composition | Size (nm) | Zeta Potential (mV) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Mean Pore Size (nm) | C-Value |
|---|---|---|---|---|---|---|---|---|
| SP-A | Porous spherical particles | Silica | 1500 | −27 | 720 | 0.8 | 2.1 | 62 |
| SP-B | Porous spherical particles | Silica (co-condensation with phenylsilane) | 1500 | −22 | 830 | 0.7 | 1.5 | 160 |
| SP-C | Porous spherical particles | Silica (co-condensation with aminosilane) | 250 | −19 | 420 | 0.4 | 2.5 | 35 |
| HNT | Hollow tubes | 1:1 (silica tetrahedral sheet, alumina sheet) | 50 × 1000 | −31 | 40 (64 *) | 0.2 | 10.3 | 57 |
| MM | Plate-shaped stacked layers | 2:1 (silica tetrahedral sheet, alumina sheet) | Stacked nanolayers of various sizes | −21 | 220–270 * | X | X | X |
| FS | Particle-aggregates forming long branched chains | Silica | 200-300* | −28 | 200 * | X | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulin-Sarfraz, T.; Kalantzopoulos, G.N.; Kvalvåg Pettersen, M.; Wold Åsli, A.; Tho, I.; Axelsson, L.; Sarfraz, J. Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study. Nanomaterials 2021, 11, 379. https://doi.org/10.3390/nano11020379
Gulin-Sarfraz T, Kalantzopoulos GN, Kvalvåg Pettersen M, Wold Åsli A, Tho I, Axelsson L, Sarfraz J. Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study. Nanomaterials. 2021; 11(2):379. https://doi.org/10.3390/nano11020379
Chicago/Turabian StyleGulin-Sarfraz, Tina, Georgios N. Kalantzopoulos, Marit Kvalvåg Pettersen, Anette Wold Åsli, Ingunn Tho, Lars Axelsson, and Jawad Sarfraz. 2021. "Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study" Nanomaterials 11, no. 2: 379. https://doi.org/10.3390/nano11020379
APA StyleGulin-Sarfraz, T., Kalantzopoulos, G. N., Kvalvåg Pettersen, M., Wold Åsli, A., Tho, I., Axelsson, L., & Sarfraz, J. (2021). Inorganic Nanocarriers for Encapsulation of Natural Antimicrobial Compounds for Potential Food Packaging Application: A Comparative Study. Nanomaterials, 11(2), 379. https://doi.org/10.3390/nano11020379