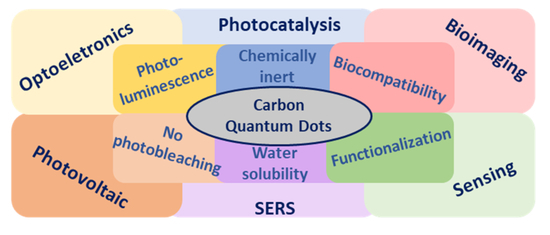
You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots
Abstract
:1. Introduction
2. Synthesis Methods of CQDs
2.1. Hydrothermal/Solvothermal Synthesis
2.2. Microwave-Assisted Synthesis
2.3. Others
2.3.1. Electrochemical Synthesis
2.3.2. Sonochemical Synthesis/Ultrasonic Treatment
2.3.3. Laser Ablation
2.3.4. Natural Sources/Biomass/Waste Products
3. Structure Modifications
3.1. Heteroatom Doping
3.1.1. Single Heteroatom Doping
N-Doped CQDs
B-Doped CQDs
3.1.2. Co-Doping Multiplex Heteroatoms
N,P-co-CQDs
N,S-co-CQDs
N,B-co-CQDs
Tri-Doped CQDs
3.2. Surface Functionalization
3.2.1. Functionalization with Small Organic Molecules
3.2.2. Functionalization with Biological Molecules
3.2.3. Functionalization with Polymers
4. Purification/Separation
4.1. Dialysis
4.2. Reversed-Phase High Performance Liquid Chromatography
4.3. Electrophoresis
4.4. Density Gradient Ultracentrifugation
4.5. Others
5. Characterization Methods
5.1. Microscopy/Diffraction
5.1.1. Transmission and Scanning Electron Microscopies (TEM and SEM)
5.1.2. Atomic Force Microscopy (AFM)
5.1.3. X-ray Diffraction (XRD)
5.2. Spectroscopy
5.2.1. Ultraviolet-Visible (UV-vis) Spectroscopy
5.2.2. Photoluminescence (PL) Spectroscopy
5.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
5.2.4. Raman Spectroscopy (RS)
5.2.5. X-ray Photoelectron Spectroscopy (XPS)
5.2.6. Energy Dispersive Spectroscopy (EDS)
6. Applications
6.1. Biomedical
6.1.1. Bioimaging
6.1.2. Drug Delivery
6.1.3. Gene Therapy/Delivery
6.1.4. Others
6.2. Chemical Sensing
6.2.1. Biological Molecules
6.2.2. Ion Sensing
6.3. Optoelectronics
6.3.1. Light-Emitting Diodes (LEDs)
6.3.2. Solar Energy Conversion
6.4. Photocatalysis
6.5. Antimicrobial and Antiviral Activity
6.5.1. Antimicrobial Activity
6.5.2. Antiviral Activity
7. Pharmacokinetics, Pharmacodynamics, and Toxicity
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Mikrochim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Wu, Y.F.; Wu, H.C.; Kuan, C.H.; Lin, C.J.; Wang, L.W.; Chang, C.W.; Wang, T.W. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef]
- Wang, W.; Damm, C.; Walter, J.; Nacken, T.; Peukert, W. Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis. Phys. Chem. Chem. Phys. 2015, 18. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications. Nanomicro Lett. 2018, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Hassan, M.; Gomes, V.G.; Dehghani, A.; Ardekani, S.M. Engineering carbon quantum dots for photomediated theranostics. Nano Res. 2017, 11, 1–41. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 10–1007. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreenivasan Sreeprasad, T.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent Advances in Synthesis, Optical Properties, and Biomedical Applications of Carbon Dots. ACS Appl. Bio Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Baragau, I.-A.; Power, N.P.; Morgan, D.J.; Heil, T.; Lobo, R.A.; Roberts, C.S.; Titirici, M.-M.; Dunn, S.; Kellici, S. Continuous hydrothermal flow synthesis of blue-luminescent, excitation-independent nitrogen-doped carbon quantum dots as nanosensors. J. Mater. Chem. A 2020, 8, 3270–3279. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Ding, J.; Zhao, L.; Zhou, T.; Ding, H.; Chen, Y.; Ding, L. Microwave-assisted synthesis of highly luminescent N-and S-co-doped carbon dots as a ratiometric fluorescent probe for levofloxacin. Mikrochim. Acta 2018, 185, 104. [Google Scholar] [CrossRef]
- Das, A.; Snee, P.T. Synthetic Developments of Nontoxic Quantum Dots. ChemPhysChem 2016, 17, 598–617. [Google Scholar] [CrossRef]
- Leong, C.R.; Tong, W.Y.; Tan, W.-N.; Tumin, N.D.; Yusof, F.A.M.; Yacob, L.S.; Rosli, M.I.H.B.; Md Abu, T. Synthesis of curcumin quantum dots and their antimicrobial activity on necrotizing fasciitis causing bacteria. Mater. Today Proc. 2020, 31, 31–35. [Google Scholar] [CrossRef]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser Ablated Carbon Nanodots for Light Emission. Nanoscale Res. Lett. 2016, 11, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Nguyen, V.C.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent lignin hybridized carbon quantum dots composites for bioimaging applications. Int. J. Biol. Macromol. 2019, 122, 954–961. [Google Scholar] [CrossRef]
- Qian, J.; Quan, F.; Zhao, F.; Wu, C.; Wang, Z.; Zhou, L. Aconitic acid derived carbon dots: Conjugated interaction for the detection of folic acid and fluorescence targeted imaging of folate receptor overexpressed cancer cells. Sens. Actuators B Chem. 2018, 262, 444–451. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, L.; Wang, H.; Song, Y.; Liu, Y.; Li, H.; Shao, M.; Huang, H.; Kang, Z. One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth. Nanoscale 2018, 10, 12734–12742. [Google Scholar] [CrossRef]
- Prathumsuwan, T.; Jamnongsong, S.; Sampattavanich, S.; Paoprasert, P. Preparation of carbon dots from succinic acid and glycerol as ferrous ion and hydrogen peroxide dual-mode sensors and for cell imaging. Opt. Mater. 2018, 86, 517–529. [Google Scholar] [CrossRef]
- Khan, Z.M.S.H.; Saifi, S.; Shumaila Aslam, Z.; Khan, S.A.; Zulfequar, M. A facile one step hydrothermal synthesis of carbon quantum dots for label-free fluorescence sensing approach to detect picric acid in aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112201. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Uriarte, D.; Domini, C.; Garrido, M. New carbon dots based on glycerol and urea and its application in the determination of tetracycline in urine samples. Talanta 2019, 201, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, L.; Wang, H.; Zhao, W.; Li, Z.; Qu, Z.; Li, J.; Sun, T.; Wang, T.; Sui, G. Synthesis of dual functional gallic-acid-based carbon dots for bioimaging and antitumor therapy. Biomater. Sci. 2019, 7, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, V.; Wang, W.; Damm, C.; Wawra, S.; Thoma, M.; Peukert, W. Microwave-assisted one-step synthesis of white light-emitting carbon dot suspensions. Opt. Mater. 2018, 80, 110–119. [Google Scholar] [CrossRef]
- Seifikar, F.; Azizian, S.; Sillanpää, M. Microwave-assisted synthesis of carbon powder for rapid dye removal. Mater. Chem. Phys. 2020, 250, 123057. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Zhang, H.; Zhang, X.; Zhuang, J.; Liu, Y.; Hu, C.; Lei, B. Far-Red Carbon Dots as Efficient Light-Harvesting Agents for Enhanced Photosynthesis. ACS Appl. Mater. Interfaces 2020, 12, 21009–21019. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Ying, Y.-L.; Hua, X.; Niu, Y.; Xu, Y.; Long, Y.-T. Electrochemically generated green-fluorescent N-doped carbon quantum dots for facile monitoring alkaline phosphatase activity based on the Fe3+-mediating ON-OFF-ON-OFF fluorescence principle. Carbon 2018, 127, 340–348. [Google Scholar] [CrossRef]
- Huang, S.; Yao, J.; Chu, X.; Ning, G.; Zhou, Z.; Liu, Y.; Xiao, Q. A ratiometric fluorescent assay for evaluation of alkaline phosphatase activity based on ionic liquid-functionalized carbon dots. Mikrochim. Acta 2020, 187, 271. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- He, C.; Yan, H.; Li, X.; Wang, X. In situ fabrication of carbon dots-based lubricants using a facile ultrasonic approach. Green Chem. 2019, 21, 2279–2285. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, L. One-step sonochemical synthesis of versatile nitrogen-doped carbon quantum dots for sensitive detection of Fe (2+) ions and temperature in vitro. Mater. Sci. Eng. C 2019, 101, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Amans, D.; Diouf, M.; Lam, J.; Ledoux, G.; Dujardin, C. Origin of the nano-carbon allotropes in pulsed laser ablation in liquids synthesis. J. Colloid Interface Sci. 2017, 489, 114–125. [Google Scholar] [CrossRef]
- Isnaeni, I.; Suliyanti, M.M.; Shiddiq, M.; Sambudi, N.S. Optical Properties of Toluene-soluble Carbon Dots Prepared from Laser-ablated Coconut Fiber. Makara J. Sci. 2019, 23, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Tejwan, N.; Saha, S.K.; Das, J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020, 275, 102046. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.; Gao, N.; Zhang, X. Synthesis of N-Doped Micropore Carbon Quantum Dots with High Quantum Yield and Dual-Wavelength Photoluminescence Emission from Biomass for Cellular Imaging. Nanomaterials 2019, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Devi, P.; Thakur, A.; Bhardwaj, S.K.; Saini, S.; Rajput, P.; Kumar, P. Metal ion sensing and light activated antimicrobial activity of aloe-vera derived carbon dots. J. Mater. Sci. Mater. Electron. 2018, 29, 17254–17261. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B 2019, 191, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef]
- Lu, W.; Gong, X.; Nan, M.; Liu, Y.; Shuang, S.; Dong, C. Comparative study for N and S doped carbon dots: Synthesis, characterization and applications for Fe (3+) probe and cellular imaging. Anal. Chim. Acta 2015, 898, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Y.; Gao, C.; Wei, J.; Zhou, H.; Chen, Y.; Dong, C.; Sreeprasad, T.S.; Li, N.; Xia, Z. Synthesis, mechanistic investigation, and application of photoluminescent sulfur and nitrogen co-doped carbon dots. J. Mater. Chem. C 2015, 3, 9885–9893. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Hua, J.; Yang, J.; Yang, Y. Hydrothermal Synthesis of Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probes for the Detection of Dopamine. J. Fluoresc. 2018, 28, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Jiao, Y.; Zhang, Y.; Li, Y.; Gao, Y.; Lu, W.; Liu, Y.; Shuang, S.; Dong, C. Multi-sensing function integrated nitrogen-doped fluorescent carbon dots as the platform toward multi-mode detection and bioimaging. Talanta 2020, 210, 120653. [Google Scholar] [CrossRef]
- Bandi, R.; Dadigala, R.; Gangapuram, B.R.; Sabir, F.K.; Alle, M.; Lee, S.H.; Guttena, V. N-Doped carbon dots with pH-sensitive emission, and their application to simultaneous fluorometric determination of iron (III) and copper (II). Mikrochim. Acta 2019, 187, 30. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Chai, L.; Ma, J.; Qian, Z.; Chen, J.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Ji, Z.; Fan, J. Sensitive detection of amoxicillin in aqueous solution with novel fluorescent probes containing boron-doped carbon quantum dots. J. Mol. Liq. 2020, 311, 113278. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Li, Y.; Zeng, Q.; Jiang, X.; Cheng, Z. Boron doped carbon dots as a multifunctional fluorescent probe for sorbate and vitamin B12. Mikrochim. Acta 2019, 186, 84. [Google Scholar] [CrossRef]
- Hu, Q.; Li, T.; Gao, L.; Gong, X.; Rao, S.; Fang, W.; Gu, R.; Yang, Z. Ultrafast and Energy-saving Synthesis of Nitrogen and Chlorine Co-doped Carbon Nanodots via Neutralization Heat for Selective Detection of Cr (VI) in Aqueous Phase. Sensors 2018, 18, 3416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Wang, Y.; Xiao, Y.; Liu, W. Hydrothermal synthesis of carbon dots codoped with nitrogen and phosphorus as a turn-on fluorescent probe for cadmium (II). Mikrochim. Acta 2019, 186, 147. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.M. Highly passivated phosphorous and nitrogen co-doped carbon quantum dots and fluorometric assay for detection of copper ions. Anal. Bioanal. Chem. 2018, 410, 6331–6336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, X.; Dong, W.; Zhou, R.; Shuang, S.; Dong, C. Nitrogen and phosphorus dual-doped carbon dots as a label-free sensor for Curcumin determination in real sample and cellular imaging. Talanta 2018, 183, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-W.; Ma, D.-K.; Wang, W.; Chen, J.-J.; Zhou, L.; Zheng, Y.-Z.; Yu, K.; Huang, S.-M. N, S co-doped carbon dots with orange luminescence synthesized through polymerization and carbonization reaction of amino acids. Appl. Surf. Sci. 2015, 342, 136–143. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Huang, C. Development of nitrogen and sulfur-doped carbon dots for cellular imaging. J. Pharm. Anal. 2019, 9, 127–132. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Yang, X.; Han, X.; Jiao, Y.; Wei, T.; Yang, D.; Xu, H.; Nie, G. Highly Fluorescent Chiral N-S-Doped Carbon Dots from Cysteine: Affecting Cellular Energy Metabolism. Angew. Chem. Int. Ed. Engl. 2018, 57, 2377–2382. [Google Scholar] [CrossRef]
- Ding, C.; Deng, Z.; Chen, J.; Jin, Y. One-step microwave synthesis of N, S co-doped carbon dots from 1,6-hexanediamine dihydrochloride for cell imaging and ion detection. Colloids Surf. B Biointerfaces 2020, 189, 110838. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Shi, D.; Zheng, T.; Wang, Y.; Zhou, X.; Chen, L. N, B-doped carbon dots as a sensitive fluorescence probe for Hg (2+) ions and 2,4,6-trinitrophenol detection for bioimaging. J. Photochem. Photobiol. B 2016, 162, 1–13. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, D.; Mo, G.; Feng, J.; Zheng, X.; Deng, B. Facile Preparation of Boron and Nitrogen Codoped Green Emission Carbon Quantum Dots for Detection of Permanganate and Captopril. Anal. Chem. 2019, 91, 11455–11460. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Carbon dots doped with nitrogen and boron as ultrasensitive fluorescent probes for determination of alpha-glucosidase activity and its inhibitors in water samples and living cells. Mikrochim. Acta 2018, 185, 394. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R. A strategy for visual optical determination of glucose based on a smartphone device using fluorescent boron-doped carbon nanoparticles as a light-up probe. Mikrochim. Acta 2019, 187, 14. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, M.; Xu, K.; Tang, M.; Lin, X.; Hu, S.; Ren, X. Facile fabrication of N/S/P tri-doped carbon dots for tetracycline detection by an internal filtering effect of a two-way matching strategy. Anal. Methods 2020, 12, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Xiao, Q. Nitrogen, phosphorus and sulfur tri-doped carbon dots are specific and sensitive fluorescent probes for determination of chromium (VI) in water samples and in living cells. Mikrochim. Acta 2019, 186, 851. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Red emission nitrogen, boron, sulfur co-doped carbon dots for “on-off-on” fluorescent mode detection of Ag (+) ions and l-cysteine in complex biological fluids and living cells. Anal. Chim. Acta 2018, 1035, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef]
- Gao, M.X.; Yang, L.; Zheng, Y.; Yang, X.X.; Zou, H.Y.; Han, J.; Liu, Z.X.; Li, Y.F.; Huang, C.Z. “Click” on Alkynylated Carbon Quantum Dots: An Efficient Surface Functionalization for Specific Biosensing and Bioimaging. Chemistry 2017, 23, 2171–2178. [Google Scholar] [CrossRef]
- Dimos, K. Tuning Carbon Dots’ Optoelectronic Properties with Polymers. Polymers 2018, 10, 1312. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, T.; Kim, J.O.; Jang, S.; Kim, D.P.; Kang, I.K.; Park, S.Y. Multifaceted thermoresponsive poly (N-vinylcaprolactam) coupled with carbon dots for biomedical applications. Mater. Sci. Eng. C 2016, 61, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 14, 2655. [Google Scholar] [CrossRef] [Green Version]
- Ostadhossein, F.; Vulugundam, G.; Misra, S.K.; Srivastava, I.; Pan, D. Chirality Inversion on the Carbon Dot Surface via Covalent Surface Conjugation of Cyclic Alpha-Amino Acid Capping Agents. Bioconjug. Chem. 2018, 29, 3913–3922. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Bioinspired thiol functionalized carbon dots for rapid detection of lead (II) ions in human serum. Opt. Mater. 2020, 99, 109514. [Google Scholar] [CrossRef]
- Rossini, E.L.; Milani, M.I.; Pezza, H.R. Green synthesis of fluorescent carbon dots for determination of glucose in biofluids using a paper platform. Talanta 2019, 201, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.Z.; Wibowo, D.L.N.; Sakti, S.C.W.; Lee, H.V. Human serum albumin capsulated hydrophobic carbon nanodots as staining agent on HeLa tumor cell. Mater. Chem. Phys. 2020, 239, 122266. [Google Scholar] [CrossRef]
- Sahu, V.; Khan, F. Synthesis of bovine serum albumin capped boron-doped carbon dots for sensitive and selective detection of Pb (II) ion. Heliyon 2020, 6, e03957. [Google Scholar] [CrossRef]
- Yang, T.; Huang, J.L.; Wang, Y.T.; Zheng, A.Q.; Shu, Y.; Wang, J.H. β-Cyclodextrin-Decorated Carbon Dots Serve as Nanocarriers for Targeted Drug Delivery and Controlled Release. ChemNanoMat 2019, 5, 479–487. [Google Scholar] [CrossRef]
- Chen, Q.; Man, H.; Zhu, L.; Guo, Z.; Wang, X.; Tu, J.; Jin, G.; Lou, J.; Zhang, L.; Ci, L. Enhanced plant antioxidant capacity and biodegradation of phenol by immobilizing peroxidase on amphoteric nitrogen-doped carbon dots. Catal. Commun. 2020, 134, 105847. [Google Scholar] [CrossRef]
- He, X.; Chen, P.; Zhang, J.; Luo, T.Y.; Wang, H.J.; Liu, Y.H.; Yu, X.Q. Cationic polymer-derived carbon dots for enhanced gene delivery and cell imaging. Biomater. Sci. 2019, 7, 1940–1948. [Google Scholar] [CrossRef]
- Janus, L.; Piatkowski, M.; Radwan-Praglowska, A.J. Microwave-Assisted Synthesis and Characterization of Poly (L-lysine)-Based Polymer/Carbon Quantum Dot Nanomaterials for Biomedical Purposes. Materials 2019, 12, 3825. [Google Scholar] [CrossRef] [Green Version]
- Arsalani, N.; Nezhad-Mokhtari, P.; Jabbari, E. Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhao, F.; Zhang, T.; Lü, C. A facile method to prepare polymer functionalized carbon dots inspired by the mussel chemistry for LED application. Dyes Pigments 2019, 162, 845–854. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Tsai, Y.-H.; Chang, C.-W. Evaluation of the dialysis time required for carbon dots by HPLC and the properties of carbon dots after HPLC fractionation. New J. Chem. 2019, 43, 6153–6159. [Google Scholar] [CrossRef]
- Vinci, J.C.; Colon, L.A. Fractionation of carbon-based nanomaterials by anion-exchange HPLC. Anal. Chem. 2012, 84, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Z. Study of chromatographic fractions from carbon dots isolated by column chromatography and a binary gradient elution via RP-HPLC. Anal. Methods 2019, 11, 760–766. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Bakal, A.A.; Shpuntova, D.V.; Kostritskiy, A.Y.; Beloglazova, N.V.; De Saeger, S.; Sukhorukov, G.B.; Sapelkin, A.V.; Goryacheva, I.Y. Gel electrophoresis separation and origins of light emission in fluorophores prepared from citric acid and ethylenediamine. Sci. Rep. 2019, 9, 14665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Kumar, A.; Ma, J.; Kuang, Y.; Luo, L.; Sun, X. Density gradient ultracentrifugation for colloidal nanostructures separation and investigation. Sci. Bull. 2018, 63, 645–662. [Google Scholar] [CrossRef] [Green Version]
- Beiraghi, A.; Najibi-Gehraz, S.A. Purification and Fractionation of Carbon Dots using pH-controlled Cloud Point Extraction Technique. J. Nanostruct. 2020, 10, 107–118. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Devendiran, M.; Kim, T.H.; Lee, I.-H. A convenient method for isolating carbon quantum dots in high yield as an alternative to the dialysis process and the fabrication of a full-band UV blocking polymer film. New J. Chem. 2018, 42, 18312–18317. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Prikhozhdenko, E.S.; Tarakina, N.V.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. Dispersion of optical and structural properties in gel column separated carbon nanoparticles. Carbon 2018, 127, 541–547. [Google Scholar] [CrossRef]
- Hinterberger, V.; Damm, C.; Haines, P.; Guldi, D.M.; Peukert, W. Purification and structural elucidation of carbon dots by column chromatography. Nanoscale 2019, 11, 8464–8474. [Google Scholar] [CrossRef] [PubMed]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Colon, L.A. Hidden Properties of Carbon Dots Revealed After HPLC Fractionation. J. Phys. Chem. Lett. 2013, 4, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Somani, D.P.; Umeno, M. Importance of Transmission Electron Microscopy for Carbon Nanomaterials Research. Mod. Res. Educ. Top. Microsc. 2007, 3, 634–642. [Google Scholar]
- Choudhary, O.P.; Choudhary, P. Scanning Electron Microscope: Advantages and Disadvantages in Imaging Components. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1877–1882. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.; Abdullah, A.; Chehimi, M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer-carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- García, R. Dynamic atomic force microscopy methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Muller, D.J.; Dufrene, Y.F. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. In Nanoscience and Technology; World Scientific: Singapore, 2009; pp. 269–277. [Google Scholar] [CrossRef]
- Huang, J.J.; Zhong, Z.F.; Rong, M.Z.; Zhou, X.; Chen, X.D.; Zhang, M.Q. An easy approach of preparing strongly luminescent carbon dots and their polymer based composites for enhancing solar cell efficiency. Carbon 2014, 70, 190–198. [Google Scholar] [CrossRef]
- Bishnoi, A.; Kumar, S.; Joshi, N. Wide-Angle X-ray Diffraction (WXRD). In Microscopy Methods in Nanomaterials Characterization; Thomas, S., Thomas, R., Zachariah, A.K., Mishra, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–337. [Google Scholar]
- Hu, Q.; Paau, M.C.; Zhang, Y.; Gong, X.; Zhang, L.; Lu, D.; Liu, Y.; Liu, Q.; Yao, J.; Choi, M.M.F. Green synthesis of fluorescent nitrogen/sulfur-doped carbon dots and investigation of their properties by HPLC coupled with mass spectrometry. RSC Adv. 2014, 4, 18065–18073. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.-S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782. [Google Scholar] [CrossRef]
- Kundu, A.; Lee, J.; Park, B.; Ray, C.; Sankar, K.V.; Kim, W.S.; Lee, S.H.; Cho, I.J.; Jun, S.C. Facile approach to synthesize highly fluorescent multicolor emissive carbon dots via surface functionalization for cellular imaging. J. Colloid Interface Sci. 2018, 513, 505–514. [Google Scholar] [CrossRef]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-Dependent Photocatalytic Activity of Carbon Dots with Surface-State Determined Photoluminescence. Appl. Catal. B 2019, 248, 157–166. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, S.; Li, D.; Zhu, C.; Ren, W.; Dong, S.; Wang, E. Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. ACS Nano 2012, 6, 400–409. [Google Scholar] [CrossRef]
- Tougaard, S. Surface analysis X-ray Photoelectron Spectroscopy. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Surface and Material Characterization Techniques. In Surface Treatment of Materials for Adhesive Bonding; Ebnesajjad, S., Ed.; William Andrew: Oxford, UK, 2014; pp. 39–75. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sens. Actuators B Chem. 2019, 286, 206–213. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Xiao, N.; Liu, S.G.; Han, L.; Li, N.B.; Luo, H.Q. pH-induced aggregation of hydrophilic carbon dots for fluorescence detection of acidic amino acid and intracellular pH imaging. Mater. Sci. Eng. C 2020, 108, 110401. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, X.; Zhang, M.; Xiao, C.; Cai, L.; Khan, W.A.; Yu, K.; Cui, J.; He, L. Blood compatible heteratom-doped carbon dots for bio-imaging of human umbilical vein endothelial cells. Chin. Chem. Lett. 2020, 31, 769–773. [Google Scholar] [CrossRef]
- Shi, X.; Hu, Y.; Meng, H.-M.; Yang, J.; Qu, L.; Zhang, X.-B.; Li, Z. Red emissive carbon dots with dual targetability for imaging polarity in living cells. Sens. Actuators B Chem. 2020, 306, 127582. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Liu, L.; Kong, Y.; Zhang, A.; Xu, K.; Han, C. The Cost-Effective Preparation of Green Fluorescent Carbon Dots for Bioimaging and Enhanced Intracellular Drug Delivery. Nanoscale Res. Lett. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, M.; Tian, L.; Qiu, Y.; Yu, Q.; Wang, X.; Guo, R.; He, Q. Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int. J. Pharm. 2020, 578, 119122. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Ma, L.; Zhang, B.; Zhang, Y.; Li, X.; Wang, T.; Zhang, W.; Li, Y.; Sang, S. Construction and application of targeted drug delivery system based on hyaluronic acid and heparin functionalised carbon dots. Colloids Surf. B Biointerfaces 2020, 188, 110768. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Gao, F.; Yu, C.; Zeng, M.; He, G.; Wu, Y.; Su, Y.; Hu, N.; Zhou, Z.; Yang, Z.; et al. Dual-targeted therapy in HER2-positive breast cancer cells with the combination of carbon dots/HER3 siRNA and trastuzumab. Nanotechnology 2020, 31, 335102. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Jin, N.; Wang, Z.; Liu, L.; Meng, L.; Li, D.; Li, X.; Zhou, D.; Liu, J.; Bu, W.; et al. Osteopromotive carbon dots promote bone regeneration through the PERK-eIF2alpha-ATF4 pathway. Biomater. Sci. 2020, 8, 2840–2852. [Google Scholar] [CrossRef] [PubMed]
- Malishev, R.; Arad, E.; Bhunia, S.K.; Shaham-Niv, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Chiral modulation of amyloid beta fibrillation and cytotoxicity by enantiomeric carbon dots. Chem. Commun. 2018, 54, 7762–7765. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, J.; He, X.; Liu, Y.H.; Yu, X.Q. Hydrophobically modified carbon dots as a multifunctional platform for serum-resistant gene delivery and cell imaging. Biomater. Sci. 2020, 8, 3730–3740. [Google Scholar] [CrossRef] [PubMed]
- Ju, E.; Li, T.; Liu, Z.; Da Silva, S.R.; Wei, S.; Zhang, X.; Wang, X.; Gao, S.J. Specific Inhibition of Viral MicroRNAs by Carbon Dots-Mediated Delivery of Locked Nucleic Acids for Therapy of Virus-Induced Cancer. ACS Nano 2020, 14, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Muktha, H.; Sharath, R.; Kottam, N.; Smrithi, S.P.; Samrat, K.; Ankitha, P. Green Synthesis of Carbon Dots and Evaluation of Its Pharmacological Activities. BioNanoScience 2020, 10, 731–744. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Yang, J.; Wu, F.-G. On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 2018, 134, 232–243. [Google Scholar] [CrossRef]
- Hua, X.-W.; Bao, Y.-W.; Wu, F.-G. Fluorescent Carbon Quantum Dots with Intrinsic Nucleolus-Targeting Capability for Nucleolus Imaging and Enhanced Cytosolic and Nuclear Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 10664–10677. [Google Scholar] [CrossRef]
- Singh, V.; Rawat, K.S.; Mishra, S.; Baghel, T.; Fatima, S.; John, A.A.; Kalleti, N.; Singh, D.; Nazir, A.; Rath, S.K.; et al. Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C elegans and BALB/c mice. J. Mater. Chem. B 2018, 6, 3366–3371. [Google Scholar] [CrossRef]
- Zuo, G.; Xie, A.; Pan, X.; Su, T.; Li, J.; Dong, W. Fluorine-Doped Cationic Carbon Dots for Efficient Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 2376–2385. [Google Scholar] [CrossRef]
- Ardekani, S.M.; Dehghani, A.; Ye, P.; Nguyen, K.-A.; Gomes, V.G. Conjugated carbon quantum dots: Potent nano-antibiotic for intracellular pathogens. J. Colloid Interface Sci. 2019, 552, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Huang, S.; Li, B.; Ashraf, U.; Li, Q.; Lu, X.; Gao, X.; Cui, M.; Imran, M.; Ye, J.; Cao, F.; et al. Quaternized Cationic Carbon Dots as Antigen Delivery Systems for Improving Humoral and Cellular Immune Responses. ACS Appl. Nano Mater. 2020, 3, 9449–9461. [Google Scholar] [CrossRef]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, L.; Sun, T.; Hou, J.; Sun, Y.; Yu, M.; Diao, Y.; Lu, S.; Zhao, W.; Wang, L. Synthesis of bifunctional carbon quantum dots for bioimaging and anti-inflammation. Nanotechnology 2020, 31, 175102. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Das, M.; Kasade, D.; Pandey, M.; Dubey, A.K.; Yadav, S.K.; Parmar, A.S. Sandalwood-derived carbon quantum dots as bioimaging tools to investigate the toxicological effects of malachite green in model organisms. Chemosphere 2020, 248, 125998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Wu, P.; Ma, C.; Wu, X.; Xu, M.; Luo, S.; Xu, Z.; Liu, S. Hydrothermal synthesis of nitrogen and boron co-doped carbon quantum dots for application in acetone and dopamine sensors and multicolor cellular imaging. Sens. Actuators B Chem. 2019, 281, 34–43. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, T.; Tian, Y. An enzyme-free amplification strategy based on two-photon fluorescent carbon dots for monitoring miR-9 in live neurons and brain tissues of Alzheimer’s disease mice. Chem. Commun. 2020, 56, 8083–8086. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, S.; Yu, Y.; Chen, F. Determination of thiourea based on the reversion of fluorescence quenching of nitrogen doped carbon dots by Hg (2). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117666. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Huang, Y.Y.; He, X.; Xie, X.F.; He, B.; Yang, J.H.; Wang, X.Y. Off-on fluorescent switching of boron-doped carbon quantum dots for ultrasensitive sensing of catechol and glutathione. Carbon 2020, 162, 234–244. [Google Scholar] [CrossRef]
- Wang, C.; Lan, Y.; Yuan, F.; Fereja, T.H.; Lou, B.; Han, S.; Li, J.; Xu, G. Chemiluminescent determination of L-cysteine with the lucigenin-carbon dot system. Mikrochim. Acta 2019, 187, 50. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, Z. Nitrogen-doped carbon quantum dots as a “turn off-on” fluorescence sensor based on the redox reaction mechanism for the sensitive detection of dopamine and alpha lipoic acid. J. Photochem. Photobiol. A Chem. 2020, 392, 112438. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Cao, L.; Zan, M.; Yang, D.; Wang, Z.; Chang, Z.; Mei, Q.; Miao, P.; Dong, W.F. Two-Step Hydrothermal Preparation of Carbon Dots for Calcium Ion Detection. ACS Appl. Mater. Interfaces 2019, 11, 44566–44572. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Lee, J.; Mehta, A.; Kumar, S.; Kim, K.H.; Basu, S.; Rawat, M. Highly fluorescent carbon dots derived from Mangifera indica leaves for selective detection of metal ions. Sci. Total Environ. 2020, 720, 137604. [Google Scholar] [CrossRef]
- Ali, H.R.H.; Hassan, A.I.; Hassan, Y.F.; El-Wekil, M.M. Development of dual function polyamine-functionalized carbon dots derived from one step green synthesis for quantitation of Cu (2+) and S (2-) ions in complicated matrices with high selectivity. Anal. Bioanal. Chem. 2020, 412, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Bidkar, A.P.; Rajasekhar, R.; Ghosh, S.S.; Mandal, T.K. A facile synthesis of nontoxic luminescent carbon dots for detection of chromium and iron in real water sample and bio-imaging. Can. J. Chem. Eng. 2019, 98, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Chen, B.B.; Zou, H.Y.; Li, Y.F.; Huang, C.Z. Inner filter with carbon quantum dots: A selective sensing platform for detection of hematin in human red cells. Biosens. Bioelectron. 2018, 100, 148–154. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Y.; Su, A.; Wang, Y. Synthesis of catalytically active carbon quantum dots and its application for colorimetric detection of glutathione. Sens. Actuators B Chem. 2018, 273, 1098–1102. [Google Scholar] [CrossRef]
- Carneiro, S.V.; De Queiroz, V.H.R.; Cruz, A.A.C.; Fechine, L.M.U.D.; Denardin, J.C.; Freire, R.M.; Do Nascimento, R.F.; Fechine, P.B.A. Sensing strategy based on Carbon Quantum Dots obtained from riboflavin for the identification of pesticides. Sens. Actuators B Chem. 2019, 301, 127149. [Google Scholar] [CrossRef]
- Chandra, S.; Singh, V.K.; Yadav, P.K.; Bano, D.; Kumar, V.; Pandey, V.K.; Talat, M.; Hasan, S.H. Mustard seeds derived fluorescent carbon quantum dots and their peroxidase-like activity for colorimetric detection of H2O2 and ascorbic acid in a real sample. Anal. Chim. Acta 2019, 1054, 145–156. [Google Scholar] [CrossRef]
- Lu, C.; Liu, J.; Gan, L.; Yang, X. Employing Cryptococcus-directed carbon dots for differentiating and detecting m-benzenediol and p-benzenediol. Sens. Actuators B Chem. 2019, 301, 127077. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, W. Hydrothermal synthesis of highly fluorescent nitrogen-doped carbon quantum dots with good biocompatibility and the application for sensing ellagic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118580. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, J.; Chen, L.; Ding, L. A novel fluorescence assay based on self-doping biomass carbon dots for rapid detection of dimethoate. J. Photochem. Photobiol. A Chem. 2020, 400, 112724. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Q.; Sun, H.; Han, J.; Pan, Y.; Yang, Z.-Q. An ultra-sensitive analytical platform based on bluish green emitting carbon quantum dots for the detection of curcumin in dietary foods. J. Food Compos. Anal. 2020, 94, 103639. [Google Scholar] [CrossRef]
- Shekarbeygi, Z.; Farhadian, N.; Khani, S.; Moradi, S.; Shahlaei, M. The effects of rose pigments extracted by different methods on the optical properties of carbon quantum dots and its efficacy in the determination of Diazinon. Microchem. J. 2020, 158, 105232. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Wang, J.; Yu, J.; Arabi, M.; Fu, L.; Li, B.; Li, J.; Chen, L. Fluorescent nanosensor designing via hybrid of carbon dots and post-imprinted polymers for the detection of ovalbumin. Talanta 2020, 211, 120727. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- Guner, T.; Yuce, H.; Tascioglu, D.; Simsek, E.; Savaci, U.; Genc, A.; Turan, S.; Demir, M.M. Optimization and performance of nitrogen-doped carbon dots as a color conversion layer for white-LED applications. Beilstein J. Nanotechnol. 2019, 10, 2004–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo-Mirasol, S.; Gené-Marimon, S.; Martínez-Ferrero, E.; Palomares, E. Inverted Hybrid Light-Emitting Diodes Using Carbon Dots as Selective Contacts: The Effect of Surface Ligands. ACS Appl. Electron. Mater. 2020, 2, 1388–1394. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Wei, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield. Nanomaterials 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-color fluorescent carbon quantum dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Padmanathan, S.; Prakasam, A. Design and fabrication of hybrid carbon dots/titanium dioxide (CDs/TiO2) photoelectrodes for highly efficient dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 3492–3499. [Google Scholar] [CrossRef]
- Zhao, H.; Benetti, D.; Tong, X.; Zhang, H.; Zhou, Y.; Liu, G.; Ma, D.; Sun, S.; Wang, Z.M.; Wang, Y.; et al. Efficient and stable tandem luminescent solar concentrators based on carbon dots and perovskite quantum dots. Nano Energy 2018, 50, 756–765. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Guo, Z.; Miao, P.; Gong, X. Carbon dots based nanocomposite thin film for highly efficient luminescent solar concentrators. Org. Electron. 2018, 62, 284–289. [Google Scholar] [CrossRef]
- Dai, D.; Tu, X.; Li, X.; Lv, T.; Han, F. Tuning solar absorption spectra via carbon quantum dots/VAE composite layer and efficiency enhancement for crystalline Si solar module. Prog. Photovolt. Res. Appl. 2019, 27, 283–289. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Yi, Z.; Chi, F.; Wang, H.; Yuan, Y.; Li, D.; Xu, K.; Zhang, X. High efficiency solar cells tailored using biomass-converted graded carbon quantum dots. Nanoscale 2019, 11, 15083–15090. [Google Scholar] [CrossRef]
- Mistry, B.; Machhi, H.K.; Vithalani, R.S.; Patel, D.S.; Modi, C.K.; Prajapati, M.; Surati, K.R.; Soni, S.S.; Jha, P.K.; Kane, S.R. Harnessing the N-dopant ratio in carbon quantum dots for enhancing the power conversion efficiency of solar cells. Sustain. Energy Fuels 2019, 3, 3182–3190. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, G.; Han, G. High-performance laminated luminescent solar concentrators based on colloidal carbon quantum dots. Nanoscale Adv. 2019, 1, 4888–4894. [Google Scholar] [CrossRef] [Green Version]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, P.; Li, X.; Luo, M.; Zhang, W.; Chen, M.; Zhou, X. Green preparation of palm powder-derived carbon dots co-doped with sulfur/chlorine and their application in visible-light photocatalysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117659. [Google Scholar] [CrossRef] [PubMed]
- Amadio, E.; Cailotto, S.; Campalani, C.; Branzi, L.; Raviola, C.; Ravelli, D.; Cattaruzza, E.; Trave, E.; Benedetti, A.; Selva, M.; et al. Precursor-Dependent Photocatalytic Activity of Carbon Dots. Molecules 2019, 25, 101. [Google Scholar]
- Ji, M.; Zhang, Z.; Xia, J.; Di, J.; Liu, Y.; Chen, R.; Yin, S.; Zhang, S.; Li, H. Enhanced photocatalytic performance of carbon quantum dots/BiOBr composite and mechanism investigation. Chin. Chem. Lett. 2018, 29, 805–810. [Google Scholar] [CrossRef]
- Kováčová, M.; Marković, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajďák, J.; Capáková, Z.; et al. Carbon Quantum Dots Modified Polyurethane Nanocomposite as Effective Photocatalytic and Antibacterial Agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Appl. Catal. B Environ. 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Wang, H.; Zeng, G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 511, 296–306. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.; Tang, J.; Shu, X.; Liu, X.; Zhang, Z.; Wang, J. Carbon quantum dots/KNbO3 hybrid composites with enhanced visible-light driven photocatalytic activity toward dye waste-water degradation and hydrogen production. Mol. Catal. 2018, 445, 1–11. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.; Qi, J.; Tong, Y.; Lv, Y.; Xu, C.; Ni, J.; Liu, W. Photocatalytic degradation of amoxicillin by carbon quantum dots modified K2Ti6O13 nanotubes: Effect of light wavelength. Chin. Chem. Lett. 2019, 30, 1214–1218. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Dai, Q.; Zhang, H.; Shen, X.; Qiu, Y.; Huang, Y.; Yu, J.; Yin, D.; Küppers, S. Wavelength-dependent effects of carbon quantum dots on the photocatalytic activity of g-C3N4 enabled by LEDs. Chem. Eng. J. 2020, 379, 122296. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, R.; Zhang, C.; Zhang, K.; Liu, W.; Shao, X.; Xue, Q.; Yue, Q. Fluorescence and photocatalytic activity of metal-free nitrogen-doped carbon quantum dots with varying nitrogen contents. Appl. Surf. Sci. 2020, 531, 147344. [Google Scholar] [CrossRef]
- Mahmood, A.; Shi, G.; Wang, Z.; Rao, Z.; Xiao, W.; Xie, X.; Sun, J. Carbon quantum dots-TiO2 nanocomposite as an efficient photocatalyst for the photodegradation of aromatic ring-containing mixed VOCs: An experimental and DFT studies of adsorption and electronic structure of the interface. J. Hazard. Mater. 2021, 401, 123402. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Song, Y.; Lu, F.; Li, H.; Wang, H.; Zhang, M.; Liu, Y.; Kang, Z. Degradable Carbon Dots from Cigarette Smoking with Broad-Spectrum Antimicrobial Activities against Drug-Resistant Bacteria. ACS Appl. Bio Mater. 2018, 1, 1871–1879. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; et al. Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 26936–26946. [Google Scholar] [CrossRef]
- Loczechin, A.; Seron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef]
- Huang, S.; Gu, J.; Ye, J.; Fang, B.; Wan, S.; Wang, C.; Ashraf, U.; Li, Q.; Wang, X.; Shao, L.; et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019, 542, 198–206. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, e1906206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.T.; Lin, H.J.; Huang, H.J.; Huang, C.C.; Lin, J.H.; Chen, L.L. Synthesis and evaluation of polyamine carbon quantum dots (CQDs) in Litopenaeus vannamei as a therapeutic agent against WSSV. Sci. Rep. 2020, 10, 7343. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, Y.; Zhou, D.; Liu, K.; Geng, N.; Lu, J.; Liu, Y.; Liu, J. Novel carbon quantum dots can serve as an excellent adjuvant for the gp85 protein vaccine against avian leukosis virus subgroup J in chickens. Poult. Sci. 2019, 98, 5315–5320. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chang, L.; Chu, H.-W.; Lin, H.-J.; Chang, P.-C.; Wang, R.Y.L.; Unnikrishnan, B.; Mao, J.-Y.; Chen, S.-Y.; Huang, C.-C. High Amplification of the Antiviral Activity of Curcumin through Transformation into Carbon Quantum Dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef]
- Wang, H.; Song, Z.; Gu, J.; Li, S.; Wu, Y.; Han, H. Nitrogen-Doped Carbon Quantum Dots for Preventing Biofilm Formation and Eradicating Drug-Resistant Bacteria Infection. ACS Biomater. Sci. Eng. 2019, 5, 4739–4749. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Fang, J.; Feng, X. Bio-nanoplatforms based on carbon dots conjugating with F-substituted nano-hydroxyapatite for cellular imaging. Nanoscale 2015, 7, 20033–20041. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Ai, X.; Ong, H.; Zhao, Y. Dual-Responsive Carbon Dots for Tumor Extracellular Microenvironment Triggered Targeting and Enhanced Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18732–18740. [Google Scholar] [CrossRef]
- Karthik, S.; Saha, B.; Ghosh, S.K.; Pradeep Singh, N.D. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery. Chem. Commun. 2013, 49, 10471–10473. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in vitro and in vivo pulmonary delivery of nucleic acid by carbon dot-based nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Bunker, C.E.; Sun, Y.P. Carbon Dots: Zero-Dimensional Carbon Allotrope with Unique Photoinduced Redox Characteristics. ACS Omega 2020, 5, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On-off-on fluorescent carbon dot nanosensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. ACS Appl Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, Z.; Yin, Z.; Zhou, C.; Du, F.; Zhou, S.; Chen, P.; Xie, Z. High color rendering index trichromatic white and red LEDs prepared from silane-functionalized carbon dots. J. Mater. Chem. C 2017, 5, 9629–9637. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-Doped Carbon Dots for “green” Quantum Dot Solar Cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Paulo-Mirasol, S.; Martinez-Ferrero, E.; Palomares, E. Direct white light emission from carbon nanodots (C-dots) in solution processed light emitting diodes. Nanoscale 2019, 11, 11315–11321. [Google Scholar] [CrossRef]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.-Y. One-Step Synthesis of Highly Luminescent Carbon Dots in Noncoordinating Solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Zdrazil, L.; Kalytchuk, S.; Hola, K.; Petr, M.; Zmeskal, O.; Kment, S.; Rogach, A.L.; Zboril, R. A carbon dot-based tandem luminescent solar concentrator. Nanoscale 2020, 12, 6664–6672. [Google Scholar] [CrossRef]
- Rodriguez-Padron, D.; Luque, R.; Munoz-Batista, M.J. Waste-derived Materials: Opportunities in Photocatalysis. Top. Curr. Chem. 2019, 378, 3. [Google Scholar] [CrossRef]
- Nekoueian, K.; Amiri, M.; Sillanpaa, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-based quantum particles: An electroanalytical and biomedical perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef]
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon dots: Current advances in pathogenic bacteria monitoring and prospect applications. Biosens. Bioelectron. 2020, 156, 112085. [Google Scholar] [CrossRef]
- Dong, X.; Moyer, M.M.; Yang, F.; Sun, Y.P.; Yang, L. Carbon Dots’ Antiviral Functions Against Noroviruses. Sci. Rep. 2017, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mozafari, M. Biological Response to Carbon-Family Nanomaterials: Interactions at the Nano-Bio Interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Kashyap, S.; Yadav, U.; Srivastava, A.; Singh, A.V.; Singh, R.K.; Singh, S.K.; Saxena, P.S. Nitrogen doped carbon quantum dots demonstrate no toxicity under in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol. Res. 2019, 8, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Pan, J.; Bai, A.M.; Hu, Y.J. Insights into the interaction of human serum albumin and carbon dots: Hydrothermal synthesis and biophysical study. Int. J. Biol. Macromol. 2020, 149, 1118–1129. [Google Scholar] [CrossRef]
- Yan, G.H.; Song, Z.M.; Liu, Y.Y.; Su, Q.; Liang, W.; Cao, A.; Sun, Y.P.; Wang, H. Effects of carbon dots surface functionalities on cellular behaviors—Mechanistic exploration for opportunities in manipulating uptake and translocation. Colloids Surf. B Biointerfaces 2019, 181, 48–57. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, I.; Sar, D.; Mukherjee, P.; Schwartz-Duval, A.S.; Huang, Z.; Jaramillo, C.; Civantos, A.; Tripathi, I.; Allain, J.P.; Bhargava, R.; et al. Enzyme-catalysed biodegradation of carbon dots follows sequential oxidation in a time dependent manner. Nanoscale 2019, 11, 8226–8236. [Google Scholar] [CrossRef] [PubMed]
















| Type of Nanodot | SQDs | CQDs | GQDs | CNDs |
|---|---|---|---|---|
| Fluorescence Scheme |  |  |  | |
| Structure | Inorganic elements in spherical crystalline structures | Mixture of sp2 and sp3 carbons in a quasi-spherical crystalline structure | Discal fragments of single nanosheets of sp2 carbons | Quasi-spherical amalgams of sp3 carbons in an amorphous structure |
| Typical Size | <6 nm | <10 nm | <20 nm | <10 nm |
| Quantum Confinement | Yes | No | ||
| Light Emission | Down-conversion PL Phosphorescence | Down-conversion PL Up-conversion PL | Down-conversion PL Up-conversion PL Phosphorescence | Down-conversion PL Up-conversion PL |
| Size dependent PL | Size dependent PL (undefined) | Size dependent PL (undefined) | Size-dependent PL | |
| Narrow PL bands | Broad PL bands | Very broad PL bands | ||
| Long PL lifetimes | Medium PL lifetimes | Short PL lifetimes | ||
| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Hydrothermal synthesis | Good production yields, ease of manipulation, good production yield of nanomaterials using high vapor pressure settings | Long synthesis duration | [21] |
| Microwave synthesis | Clean, ease of manipulation, low-temperature, and economic | Bulk metallic materials unusable due to electromagnetic field interferences | [22,23] |
| Electrochemical synthesis | Ease of operation, potential for mass production, does not involve harsh or toxic chemicals | Laborious purification processes | [24] |
| Sonochemistry synthesis | The only general method for doping CQDs using bulk metals (Ga, In, Bi, Sn, Pb, Cd, Sb, and Zn) | Prolonged synthesis process | [25] |
| Laser ablation synthesis | High quality CQDs, swift synthesis | Low reproducibility; difficulty in correlating experimental conditions with obtained CQDs properties | [26,27] |
| Biomass synthesis | Simple, cost effective, and easily available in nature | Possible environmental contamination; necessity of additional chemical modifications to increase QY | [16,28] |
| Application | Target | Ligands | Receptor | CQDs Synthesis Method | Precursors | Reaction Conditions | Modifications and Functionalization | Purification | Average Size (nm) | QY (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioimaging | HeLa cells | n/a | n/a | Hydrothermal | Citric acid ethylenediamine alkali lignin | 150 °C, 4 h | Lignin hybridized | Dialysis (3500 Da, 2 days) | <10 | 43 | [30] |
| Bioimaging | Hela, SMMC-7721 and A549 cells | Folic acid | Folate receptors | Hydrothermal | Aconitic acid Ethylenediamine | 150 °C, 5 h | N-doping | n/a | 1.6 | 56.5 | [31] |
| Growth promotion | Mung bean plant | n/a | n/a | Hydrothermal | Citric acid L-Cysteine D-Cysteine | 180 °C, 1.5 h | N, S-doping Chirality | Dialysis (500–1000 Da, 5 days) | 6 | L-CDs: 31 D-CDs: 33 | [32] |
| Bioimaging Antitumor therapy | HeLa cells (in vivo and in vitro) | Gallic acid | n/a | Microwave-assisted | Gallic acid Citric acid Ethane diamine | 700 W, 3 min | Gallic acid hybridized | Centrifugation (10,000× g rpm, time 10 min) Dialysis (500 Da, 72 h) | 2–4 | 25 | [37] |
| Bioimaging | Streptococcus sp. and Klebsiella pneumoniae | n/a | n/a | Mechanical and ultrasonic millings | Curcumin (BOSF) | Pulsed mode (750 W, 20 kHz), intensity of 30 W/cm2, 20 min | n/a | n/a | 13.7 | n/a | [26] |
| Bioimaging | HeLa cells, L02 cells, and macrophage cells | n/a | n/a | Pulsed laser ablation | Platanus biomass | Repetition rate of 10 Hz, wavelength of 1064 nm, 20 mJ, 3–6 ns, 30 min | N-doping | Centrifugation (10,000× g rpm, time n/a) × 5 | 8 | 32.4 | [50] |
| Bioimaging | E. coli, Aspergillus aculeatus and Fomitopsis sp. | n/a | n/a | Hydrothermal | Manilkara zapota fruits | blue C-dots: 100 °C, 60 min | n/a | Dialysis (12,000–14,000 Da, 24 h) | 1.9 | 5.7 | [52] |
| green C-dots: 80 °C, 30 min | 2.9 | 7.9 | |||||||||
| yellow C-dots: 80 °C, 15 min | 4.5 | 5.2 | |||||||||
| Bioimaging | SMMC7721 cells | n/a | n/a | Hydrothermal | p-Phenylenediamine Ammonia | 200 °C, 8 h | N-doping | Dialysis (500–1000 Da, 3 days) | 3.2 | 13.2 | [57] |
| Bioimaging | HEp-2 cells | n/a | n/a | Hydrothermal | Citric acid Methionine | 200 °C, 3 h | N, S-doping | Centrifugation (12,000× g rpm, 15 min) Dialysis (500 Da, 12 h) | 5 | 13.8 | [67] |
| Cellular ATP monitoring | T24 cells | n/a | n/a | Hydrothermal | L-Cysteine D-Cysteine | 60 °C, 24 h | N, S-doping Chirality | Dialysis (MWCO n/a, time n/a) | 5–7 | 41.26 | [68] |
| Bioimaging Ion detection | MCF-7 cells | n/a | n/a | Microwave-assisted | 1,6-Hexanediamine dihydrochloride Dimethylsulfoxide | 180 °C, 35 min | N, S-doping | Filtration with filter membrane (0.22 μm) Dialysis (500 Da membrane,36 h) | 4.35 | 24 | [69] |
| Bioimaging | HeLa and MCF-7 cells | n/a | n/a | Hydrothermal | 3-Aminobenzeneboronic acid 1,2-Ethylenediamine | 160 °C, 7 h | N, B-doping | n/a | 2.61 | 47.3 | [72] |
| Bioimaging Bacteria identification | Escherichia coli (E. coli), Desulfovibrio desulfuricans (D. desulfuricans), Staphylococcus sciuri (S. sciuri), Listeria monocytogenes (L. monocytogenes), Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) | 3-Aminophenylboronic acid/vancomycin hydrochloride/polymyxin B sulfate | cis-diol/(D)-Ala-(D)-Ala peptide/LPS | Hydrothermal | Ammonium citrate dibasic 3-Aminophenylboronic acid/vancomycin hydrochloride/polymyxin B sulfate | 180 ℃, 4 h | 3-Aminophenylboronic acid/vancomycin hydrochloride/polymyxin B sulfate hybridized | Centrifugation (10,000× g rpm, 15 min) Dialysis (1000 Da, overnight) | 3–6 | n/a | [118] |
| Intracellular pH imaging | HepG2 cells | n/a | n/a | Hydrothermal | Quercetin Ethylenediamine | 200 °C, 12 h | N-doping | Filtration with filter membrane (0.22 μm) Silica gel column chromatography (CH2Cl2/MeOH (V/V, 20/1)) | 2.7 | 13.4 | [119] |
| Bioimaging | HUVEC cells | n/a | n/a | Solvothermal | m-Phenylenediamine Tobias acid | 200 °C, 10 h | N, S-doping | Centrifugation (10,000× g rpm, 10 min.) Filtration with filter membrane (0.22 μm) | 2.59 | 37.2 | [120] |
| Cell polarity imaging | HepG2 cells | n/a | n/a | Hydrothermal | p-Phenylenediamine | 200 °C, 12 h | N-doping | Centrifugation (6577× g, 15 min) Silica gel column chromatography (EtOH/Ethyl acetate) | 5 | 3.40 (in water) | [121] |
| Drug delivery Bioimaging | HO-8910 cells | Doxorubicin | n/a | Carbonization | Sodium citrate dehydrate Urea | 200 °C, 1 h | N, S-doping Conjugation with doxorubicin | Centrifugation (10,000× g rpm, 10 min, 3 ×) Dialysis (1000 Da, 24 h) | 2.75 | 93 | [122] |
| Drug delivery Bioimaging | 4T1 cells | Hyaluronic acid | CD44 | Hydrothermal | Citric acid Branched-PEI | 180 °C, 1 h | Hyaluronic acid-modified Conjugation with doxorubicin | Ultrafilytration (MWCO: 50,000), 4000 rpm, 20 min, 4× | 10 | n/a | [123] |
| Drug delivery Bioimaging | MGC-803 cells | Hyaluronic acid | CD44 | Hydrothermal | Glucose Glycol + PEI | 180 °C, 3 h + 80 °C, 3 h | Hyaluronic acid-modified Conjugation with heparin | Filtration with filter membrane (0.22 μm) Centrifugation (10,000× g rpm, 20 min) | 3.9 | n/a | [124] |
| Drug delivery Bioimaging | SJGBM2, CHLA266, CHLA200 and U87 cells | Tranferrin | Tranferrin receptors | Reflux | Carbon nano powder | 110 °C, 15 hs | Triple-conjugated system (epirubicin, temozolomide and transferrin) | Centrifugation (3000× g rpm, 30 min) Dialysis (3500 Da, 5 days) | 3.5 | n/a | [125] |
| Gene therapy Bioimaging | BT-474 cells | HER3 siRNA | HER3 | Hydrothermal | Citric acid monohydrate Ethylenediamine + Branched Polyetherimide | 200 °C, 4 h | Branched PEI -functionalized N-doping | Centrifugation (13,000× g rpm, 20 min) Dialysis (1000 Da, 96 h) | 5.5 | n/a | [126] |
| Bone regeneration promotion | Pre-osteoblast cells | n/a | n/a | Microwave-assisted | Ascorbic acid PEI | 450 W, 5 min | Polymer passivation | Centrifugation (12,000× g rpm, 15 min) Filtration with filter membrane (0.22 μm) | 2–3 | n/a | [127] |
| Modulation of amyloid beta fibrillation and toxicity Bioimaging | SH-SHSY5Y cells | n/a | Aβ42 | Hydrothermal | L-lysine D-lysine | 170 °C, 2 h | Chirality | Dialysis (2000 Da, 24 h) | 3.9 | n/a | [128] |
| Photosynthesis enhancement (growth stimulation) | Chloroplasts (in vivo and in vitro) | n/a | n/a | Microwave-assisted | Glutathione Formamide | 800 W, 3 min | N, S-doping | Centrifugation (10,000× g rpm, 5 min) Dialysis (3500 Da, time n/a) | 3.8 | 18.5 | [40] |
| Gene therapy Bioimaging | A549 cells | n/a | n/a | Solvothermal | PEI 600 Da | 180 °C, 5 h | functionalization with hydrophobic chains with different degrees of substitution | Filtration with filter membrane (0.45 μm) Dialysis (1000 Da, 3 days) | 6.4 | PEI-CD: 21.49 | [129] |
| Gene therapy Bioimaging | Kaposi’s sarcoma-associated herpes virus (KSHV) cells (in vivo and in vitro) | locked nucleic acid (LNA) | Viral miRNAs | Microwave-assisted | Citric acid PEI | 1150 W, 3 min | locked nucleic acid (LNA) functionalized | Centrifugation (13,000× g rpm, 10 min) Dialysis (14,000 Da, 2 days) | 3.0 | n/a | [130] |
| Bioimaging | Fusarium oxysporum, S. aureus, P. aeruginosa, B. subtilis, E. coli, MCF-7 and HepG2 cells | n/a | n/a | Microwave-assisted | Pomegranate Watermelon extract PEG 200 | Microwave radiation, 2 min at the interval of 10 s each | Polymer passivation | n/a | 1–5 | n/a | [131] |
| Bioimaging | HeLa cells | Human serum albumin | gp60 | Pyrolysis | Tartaric acid L-tyrosine | 220 °C, 30 min | HSA modified | n/a | 5.50 | n/a | [85] |
| Drug delivery Bioimaging | HeLa and HEK 293 T cells | Doxorubicin | Folate receptors | Hydrothermal | Citric acid BPEI folic acid | 200 °C, 4 h | Polymer passivation β-cyclodextrin modified | Centrifugation (10,000× g rpm, 15 min) Dialysis (100–500 Da, 48 h) | 2.8 | 64.5 | [87] |
| Antioxidant capacity enhancement | Arabidopsis thaliana seedlings | Phenol | Horseradish peroxidase | Electrochemical | Ammonia | certain voltage with graphite electrodes; electrolyte of a certain concentration of ammonia aqueous solution; several days | N-doping Horseradish peroxidase modified (immobilization) | Centrifugation (10,000× g rpm, 15 min) Dialysis (3500 Da membrane) | n/a | n/a | [88] |
| Gene therapy Bioimaging | HeLa cells | Cy5-labelled DNA | n/a | Hydrothermal | Citric acid Pcyclen | 180 °C, 5 h | Polymer passivation | Centrifugation (12,000× g rpm, 10 min) Dialysis (1000 Da, 1 day) | 1.8 | 3.25 | [89] |
| Citric acid Ptaea | 5.4 | 1.73 | |||||||||
| Antioxidant capacity | human dermal fibroblasts | n/a | n/a | Microwave-assisted | L-lysine Propylene carbonate | 240 °C, 120 min | Polymer passivation | Dialysis (500–1000 Da, time n/a) | 2–5 | 23.3 | [90] |
| Drug delivery Bioimaging | MCF-7 cells | Methotrexate | Folate receptors | Microwave-assisted | Gelatin PEG | 600 W, 10 min | Polymer passivation | Centrifugation (12,000× g rpm, 15 min) | 6 | 34 | [91] |
| Bioimaging: Microorganism imaging and cancer/normal cells differentiation In vivo nude mice bearing U14 tumours diagnosis | Hep G2, A549, L02 and U14 and AT II cells Staphylococcus aureus, Escherichia coli, Trichoderma reesei, and Saccharomyces cerevisiae cells Zebrafish | n/a | Fe3+/Glutathione | Solvothermal | N-[3-(Trimethoxysilyl)propyl]ethylenediamine (DAMO) glycerol | degassing process with nitrogen, 2 min 260 °C, 12 h | n/a | Centrifugation (8000× g rpm, 10 min) Dialysis (~1 kDa, 2 days) | ~6.1 | 45 | [132] |
| Bioimaging Drug delivery | Nucleolus | Protoporphyrin IX (PpIX) | RNA (nucleolus) caveolae-mediated endocytosis (CvME), lipid raft-mediated endocytosis (LrME), clathrin-mediated endocytosis (CME), and macropinocytosis (cellular uptake) | Hydrothermal | m-phenylenediamine L-cysteine | 160 °C, 10 h | dicyclohexylcarbodiimide (DCC) 1-hydroxybenzotriazole (HOBt) | Centrifugation (15,000× g rpm, 15 min) Dialysis (1000 Da, 2 days) | CQs: 3.8 | ~8.4 | [133] |
| CDs-PpIX: 25.2 | 62.1 | ||||||||||
| Bioimaging in vivo | Caenorhabditis elegans (C. elegans) BALB/c mice | n/a | n/a | (i) Hydrothermal treatment (Blue-CQDs) (ii) ortho-phosphoric acid treatments (Green-CQDs) | beetroot extract (Beta vulgaris) | 150 °C, 16 h | n/a | Centrifugation (1500× g rpm, 2500 rpm and 4000 rpm, 10 min each) | (i) 5 (ii) 8 | (i) 6 (ii) 5 | [134] |
| 100 °C, 2 h | |||||||||||
| Gene delivery | HEK 293 T, NIH 3T3, COS-7, and HepG2 cells B16F10 and A549 cells Primary 3T3-L1 and mESCs cells | pDNA | Cytomembrane, Karyotheca, endosome/lysosome, membranes | Solvothermal | tetrafluoroterephthalic acid (fluorine) terephthalic acid 1.8 kDa branched-polyethyleneimine (1.8 k b-PEI) 25 kDa branched-polyethyleneimine (25 k PEI) | 180 °C, 6 h | fluorine-doped cationic CDs (FCDs): tetrafluoroterephthalic acid (fluorine) pDNA | Dialyzed (3.5 kDa, time n/a) | 4.8 (FCDs) 150–200 (FCDs/DNA) | n/a | [135] |
| Drug delivery | Porphyromonas gingivalis (P. gingivalis) | metronidazole (MET) | n/a | Hydrothermal | chlorophyll | 240 °C, 18 h | n/a | Centrifugation (5000× g, 30 min) Filtration (0.22 mm) Ultrafiltration membranes (3 kDa and 30 kDa) | 2–4 | 56% | [136] |
| Cancer detection | - | - | - | Hydrothermal (CDs) | sweet lemon peel | 180 °C, 3 h (CDs) | 1-(3- Dimethylaminopropyl)-3-ethyl carbodiimide (EDC) | Filtration Concentration rotary evaporator | 1.5 –6.5 | n/a | [137] |
| Triple negative breast cancer (TNBC) | polyamidoamine (PAMAM) dendrimers (G1, G2 and G3) | Copper (II) overexpressed in TNBC MDA-MB-231 cells | Hydrothermal (CD-PAMAM (CDP): CDP1, CDP2, CDP3 | CDs | RT, 16 h | N-hydroxysuccinimide (NHS) ethylenediamine (EDA) PAMAM dendrimers G1, G2 and G3 | Dialysis (3.5 kDa, 3 days) | n/a | |||
| Gene therapy Bioimaging agent for TNBC cells | Triple negative breast cancer (TNBC)—MDA-MB 231 cell | eGFP-pDNA | RGD receptor αvβ3 integrin | Hydrothermal (RGDS decorated CDP3 (CDP3-RGDS)) | CDP conjugated (PAMAM) dendrimers G3 (CDP3) | RT, 16 h | EDC and NHS RGDS peptide | Dialysis (3.5 kDa, 3 days) | 7–27 | n/a | |
| Skin permeability | methicillin-sensitive Staphylococcus aureus (ATCC 6538) methicillin-resistant Staphylococcus aureus (ATCC 4300) Escherichia coli (ATCC 25922) Staphylococcus epidermidis (ATCC 49134) Bacillus cereus (ATCC 14579) | n/a | n/a | Hydrothermal | polyethyleneimine (PEI) citric acid (CA) | 250 °C, 4 h | N-doping | Dialysis (≥12,000 Da, 24 h) | 70–10 | PEI:CA ratio (1:0,5)–31 (1:1)–53 (1:2)–7.6 | [138] |
| Induction of potent humoral and cellular immune responses | LnCaP cells Bone marrow-derived dendritic cells (BMDCs) Mouse leukemic monocyte macrophage cells (RAW264.7) BALB/c mice OVA-specific CD4 +T and CD8 +cytotoxic T lymphocytes (CTLs) | ovalbumin (OVA) antigen | antiOVA IgG1, IgG2a and IgG2b | Hydrothermal | N,N′-dodecyl2-hydroxyl-N,N,N′,N′-tetramethyl diammonium dichloride (BQAS) | 180 °C, 12 h | ovalbumin (OVA) | Centrifugation (10,000× g rpm,15 min) Dialysis (500 Da, 12/12 h) | 3.74 | 7.8 | [139] |
| Cancer bioimaging | 27 cancer cell lines of different origin, a side population (SP) of cancer stem-like cells isolated from MDA-MB-231 cells27, brain cancer stem cell lines (BCSCs), 12 patient-derived and 18 non-cancerous cell lines mice bearing HeLa tumours | n/a | large neutral amino acid transporter (LAT1) | Hydrothermal | LAAM TC-CQDs: 1,4,5,8-tetraminoanthraquinone (TAAQ) citric acid (CA) | 180 °C, 2 h | n/a | Silica column chromatography using mixtures of dichloromethane and methanol (10:1) as eluents for three rounds | 2.45 | 6.8 (in water) | [140] |
| Drug delivery | cancer cells and non-cancerous cells | DNA-damaging chemotherapy drugs: topotecan hydrochloride (TPTC), DOX and hydroxycamptothecin | Nuclei DNA | topotecan hydrochloride (TPTC) DOX hydroxycamptothecin | Dialysis (MWCO n/a, time n/a) | ||||||
| Brain cancer imaging and treatment | mice bearing U87 brain tumours | topotecan hydrochloride (TPTC) | LAT1 in Blood-Brain Barrier (BBB) | ||||||||
| Tumour-specific imaging and drug delivery | HeLa and CCC-ESF-1 cells | 1,4-diaminoanthraquinone (1,4-DAAQ | LAT1-mediated tumour-specific | 1,4-CQDs: 1,4-diaminoanthraquinone (1,4-DAAQ) citric acid (CA) | n/a | Silica column chromatography using mixtures of dichloromethane and methanol (20:1) as eluents | n/a | n/a | |||
| 1,5-diaminoanthraquinone (1,5-DAAQ) | 1,5-CQDs: 1,5-diaminoanthraquinone (1,5-DAAQ) citric acid (CA) | n/a | n/a | ||||||||
| 2,6-diaminoanthraquinone (2,6-DAAQ) | 2,6-CQDs: 2,6-diaminoanthraquinone (2,6-DAAQ) citric acid (CA) | n/a | n/a | ||||||||
| n/a | Solvothermal | Phe-CQDs: phenylalanine (Phe) | 180 °C in oven, 8 h | n/a | Silica column chromatography using mixtures of dichloromethane and methanol (50:1) as eluents | n/a | n/a | ||||
| Bioimaging | HeLa cells BALB/c mice | ibuprofen | n/a | Microwave- assisted | Triethanolamine Ibuprofen | 700 W, 8 min | N-doping | Centrifugation (8000× g rpm, 10 min) Dialysis (14 kDa, 3 days) | 6.99 | 22 | [141] |
| Anti-inflammatory | BALB/c mice | COX-1 or COX-2 | |||||||||
| Bioimaging | Mung beans (Vigna radiata) Human MG-63 osteosarcoma cell line Laboratory-bred strain golden hamsters | n/a | Malachite green | Hydrothermal | Sandalwood powder | 200 °C, 8 h | n/a | Centrifugation (15,000× g rpm, 20 min) Filtration (0.22 µm) | 3.5 | 12 | [142] |
| Bioimaging | Hepg2 cells | n/a | n/a | Hydrothermal | Citric acid borax p-phenylenediamine | 180 °C, 5 h | N, B-doping | Filtration (0.22 μm) Dialysis (500 Da, 24 h) | 3.53 | n/a | [143] |
| Analyte(s) | Sensing Mechanism | CQDs Synthesis Method | Precursors | Reaction Conditions | Modifications and Functionalization | Purification | Average Size (nm) | QY (%) | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Folic acid | dynamic quenching | Hydrothermal | Aconitic acid (AA) 1,2-ethylenediamine (EDA) | 150 °C, 5 h | N-doping | n/a | 1.6 | 56.5 | 1–100 µm | 40 nm | [31] |
| Fe (II) | static quenching | Hydrothermal | succinic acid glycerol | 250 °C, 6 h | n/a | Dialysis (1000 Da, 3 days) Centrifugation (10,000× g rpm, 20 min) | 2.3 | 11 | 10–50 μm | 21.9 μm 0.7 μm | [33] |
| H2O2 | 4.6 | 100–500 nm | |||||||||
| Picric acid | FRET | Hydrothermal | L-Lysine thiourea | 220 °C, 5 h | N,S-doping | Centrifugation (10,000× g rpm, 10 min) | 6.86 | 53.19 | 1–10 μm | 0.24 μm | [34] |
| Cr (VI) | IFE | Continuous hydrothermal flow | citric acid ammonia | 450 °C | N-doping | Filtration (0.22 µm alumina membrane) Dialysis (30 kDa, 1 kDa, time n/a) | 3.3 ± 0.7 | 14.91 ± 0.24 | 5–250 ppm | 0.365 ppm | [22] |
| Tetracycline | static quenching | Microwave-assisted | glycerol urea | 700 W, 4 min | N-doping | Centrifugation (3000× g rpm, 4 min) Dialysis (12,000 Da, 3 days) | 13.2 | 9.8 | 0.50–25 μm | 165 nm | [36] |
| Levofloxacin | ET | Microwave-assisted | L-cysteine ammonium citrate | 750 W, 2.5 min | N,S-doping | n/a | 2 | 64 | 0.01–70 mg L−1 | 5.1 μg L−1 | [24] |
| Alkaline phosphatase | fluorescence quenching (ON-OFF) | Electrochemical | Pyrocatechol ethanediamine | 10 V DC, 30 min | N-doping | Dialysis (3500 Da, 1 day) | 3.15 | 30.6 ± 0.12 | 5–500μm | 0.5 μm | [42] |
| Alkaline phosphatase | ratiometric fluorescence | Electrochemical | [BMIM][BF4] | 15 V DC, 4 h | Ionic liquid-functionalization | Centrifugation (10,000× g rpm, 20 min) Dialysis (1000 Da, 2 days) | 2.75 | 23.4 | 0.04–3.2 U L−1 (12 to 960 pm) | 0.012 U L−1 (3.6 pm) | [43] |
| Fe (II) | fluorescence quenching and ratiometric fluorescence | Ultrasonic | dopamine hydrochloride dimethylformamide | pulsed mode (600 W, 20 kHz) with a frequency of 1 s, 8 h | N-doping | Centrifugation (n/a rpm, time n/a) Dialysis (500 Da, time n/a) | 2.8–4.1 | 3.6 | 0–50 μm | 38 nm | [46] |
| Fe (III) | fluorescence quenching | Pyrolysis | Aloe-Vera extract | 190 °C, 20 min | n/a | Dialysis (300 Da, time n/a) | 6–8 | 12.3 | 70 ppb–10 ppm | 33 ppb | [51] |
| Dopamine | ET | Hydrothermal | polyacrylamide | 180 °C, 5 h | N-doping | Centrifugation (13,000× g rpm, 20 min) Filtration (0.22 µm) | 3 | 23.1 | 0.1–200 μm | 0.05 μm | [56] |
| Cr (VI) | IFE | Hydrothermal | p-phenylenediamine ammonia | 200 °C, 8 h | N-doping | Dialysis (500–1000 Da, 3 days) | 3.2 | 13.2 | 0.0375−3 μm | 22.5 nm | [57] |
| 2,4,6-Trinitrophenol (TNP) | 19−27 μm | 3.69 μm | |||||||||
| ascorbic acid | 5–50 μm | 3.2 μm | |||||||||
| Fe (III) | dynamic quenching | Hydrothermal | o-phenylenediamine (OPD) 2,5 pyridinedicarboxylic acid (2,5 PDC) | 180 °C, 5 h | N-doping | Centrifugation (n/a rpm, time n/a) Filtration (0.22 µm) Dialysis (3500 Da, 1 day) | 4.7 | 47 | 3–60 μm | 0.31 μm | [58] |
| Cu (II) | 0.5–15 μm | 56 nm | |||||||||
| Amoxicillin | fluorescence enhancement | Hydrothermal | citric acid boric acid | 210 °C, 24 h | B-doping | Filtration (0.22 µm) | 2.3 | 30.85 | 1.43–429.12 μm | 0.825 μm | [60] |
| Potassium sorbate | FRET | Hydrothermal | phenylboronic acid | 200 °C, 10 h | B-doping | Centrifugation (7060× g, 15 min) Filtration (0.22 µm) Dialysis (100 Da, 48 h) | 3.3 | 12 | 0.20–24 μm | 6.1 nm | [61] |
| Vitamin B12 | 0.20–30 μm | 8.0 nm | |||||||||
| Cd (II) | fluorescence enhancement | Hydrothermal | o-phosphorylethanolamine citric acid | 180 °C, 12 h | N,P-doping | Dialysis (1000 Da, overnight) Filtration (0.22 µm) | 1.6 | 8.17 | 0.5–12.5 μm | 0.16 μm | [63] |
| Cu (II) | IFE | Hydrothermal | Glucose concentrated H3PO4 polyethylene glycol diamine | 90–100 °C, 9 h | N,P-doping | Centrifugation (14,000× g rpm, 30 min) Dialysis (1000 Da, 1 day) | 3.5 | 25 | 4×10−9–4 × 10−7 M | 1.5 nm | [64] |
| Curcumin | IFE | Self-exothermic reaction | Glucose 1,2-ethylenediamine concentrated phosphoric acid | acid-base neutralization spontaneous heat,6 min | N,P-doping | Dialysis (1000 Da, 3 days) | 3.5 ± 0.2 | 9.59 | 0.5–20 µm | 58 nm | [65] |
| MnO4- | fluorescence quenching | Microwave-assisted | 1,6-hexanediamine dihydrochloride dimethyl sulfoxide | 180 °C, 35 min | N,S-doping | Filtration (0.22 µm) Dialysis (500 Da, 36 h) | 4.35 | 24 | 1–20 μm | 0.34 μm | [69] |
| Cr2O72- | Linearity not satisfied | 0.23 μm | |||||||||
| Permanganate | static quenching | Hydrothermal | p-amino salicylic acid boric acid ethylene glycol dimethacrylate | 180 °C, 5 h | N,B-doping | Centrifugation (12,000× g rpm, 15 min) Filtration (0.22 µm) Dialysis (1000 Da, 1 day) | 5 | 19.6 | 0.05–60 μm | 13 nm | [71] |
| Captopril | 0.1–60 μm | 0.03 μm | |||||||||
| Glucose | aggregation-induced emission (AIE) | Hydrothermal | 4-carboxyphenylboronic acid o-phenylenediamine rhodamine B | 150 °C, 5 h | N,B-doping | Centrifugation (rpm n/a, time n/a) | 30 | 46 | 32 μm–2 mm | 8 μm | [73] |
| Tetracycline | IFE | Ultrasonic | thiamine pyrophosphate | alkaline solution, room temperature, 240 min | N,S,P-doping | Dialysis (2000 Da, 48 h) | 3.19 | 20.5 | 0.1−20 μm | 0.0444 μm | [74] |
| Cr (VI) | IFE | Hydrothermal | p-aminobenzenesulfonic acid tetrakis(hydroxymethyl)phosphonium chloride | 180 °C, 10 h | N,S,P-doping | Filtration (0.22 µm) Dialysis (500 Da, 72 h) | 2.18 | 36.8 | 1–500 μm | 0.23 μm | [75] |
| miRNA-9 | fluorescence enhancement | Hydrothermal | Thiourea o-phenylenediamine | 200 °C, 8 h | N-doping | Filtration (0.22 µm) Dialysis (MWCO n/s, time n/a) | 3.6 ± 0.5 | n/a | 4.00–250 fm | 0.57 ± 0.12 fm | [144] |
| Thiourea | static quenching (ON-OFF) | Hydrothermal | ammonium citrate dextrin | 165 °C, 5.5 h | N-doping | Filtration (0.22 µm) | 1 | 17 | 0.90–10.0 μm | 0.15 μm | [145] |
| Catechol | photoelectron transfer | Hydrothermal | citric acid sodium tetraphenylborate | 180 °C, 8 h | B-doping | Extraction method with CCl4/H2O | 8 | 42 | 1–50 nm | 0.25 nm | [146] |
| Glutathione | 2–100 nm | 0.5 nm | |||||||||
| L-cysteine | Chemiluminescence enhancement | Hydrothermal | Starch | 190 °C, 2 h | n/a | Centrifugation (15,000× g rpm, 20 min) | 3.2 | n/a | 10.0–100 μm | 8.8 μm | [147] |
| Dopamine | fluorescence quenching | Hydrothermal | anhydrous citric acid ethylenediamine | 150 °C, 2 h | N-doping | Dialysis (3000 Da, 2 days) | n/a | n/a | 0.05–15 μm | 0.035 μm | [148] |
| α-lipoic acid | 0.5−55 μm | 0.39 μm | |||||||||
| Ca (II) | static quenching | Hydrothermal | 1st) citric acid and ethylenediamine 2nd) thylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) | 180 °C, 8 h + 150 °C, 4 h | EGTA functionalized | Centrifugation (10,000× g rpm, time n/a) Dialysis (500 Da, 1 day) | 5 | n/a | 15−300 μm | 0.387 μm | [149] |
| Fe (II) | fluorescence quenching | Pyrolysis | mango (Mangifera indica) leaves | 300 °C, 3 h | n/a | Vacuum filtration | 2 to 10 | 18.2 | 0–1000 μL of 10 ppm Fe (II) | 0.62 ppm | [150] |
| Cu (II) | static quenching | Pyrolysis | Vitis vinifera grape juice | 200°C, 6 h | Polyamine functionalized | Filtration (0.45 µm) | 8 | 32.1 | 0.07–60 μm | 0.02 μm | [151] |
| S2- | 0.8−95 μm | 0.24 μm | |||||||||
| Cr (VI) | dynamic and static quenching | Hydrothermal | Potato | 150 °C, 90 min 200 °C, 2.5 h | n/a | Filtration Paper filtration (11 µm) Centrifugation (12,000× g rpm, 10 min) | 5.97 | 6.08 | 0.5−100 μm | 0.012 μm | [152] |
| Fe (III) | 0.5−5 μm | 0.000549 μm | |||||||||
| Pb (II) | ET | Hydrothermal | L-lysine L-glutathione | 190 °C, 24 h | Thiol-functionalized | Filtration Centrifugation (8000× g rpm, 20 min) | 5 | n/a | 0−20 μm | 2.2 μm | [83] |
| Glucose | fluorescence quenching | Microwave-assisted | citric acid tyramine | 800 W, 120 s | N-doping; Tyramine hybridized | Centrifugation (9000× g rpm, 15 min) Filtration (0.45 µm) | 17.5 | 11.0 ± 0.8 | 10−6–10−5 M | n/a | [84] |
| Pb (II) | static quenching | Microwave-assisted | Urea citric acid boric acid | 700 W, 4 min | N,B-doping; BSA modified | Dialysis (500 Da membrane, 1 day) | <5 | n/a | 1−10 ppb | 0.08 ppb | [86] |
| Fe (III) | fluorescence static quenching | Solvothermal | Glycerol N-[3-(trimethoxysilyl)propyl]ethylenediamine (DAMO) | degassing process with nitrogen, 2 min 260 °C, 12 h | Silicon and Nitrogen co-doping | Centrifugation (8000× g rpm, 10 min) Dialysis (~1 kDa, 2 days) | ~6.1 | 45 | 0.1–100 µm | 16 nm | [132] |
| Fe3+ | Fluorescent quenching | Hydrothermal | Thiamine hydrochloride (Vitamin B1) ethylenediamine | 200 °C, 12 h | S-doping | Centrifugation (rpm n/a, time n/a) Dialysis (3500 Da, 3 days) | 3.2 | 4.4 | 0.1−1.0 mm | 177 nm | [153] |
| tetracycline | Fluorescent static quenching | Microwave-assisted | Xylan NH4OH | 200 °C, 200 W, 10 min | N-doping | Filtration (0.22 μm) | 7.89 | 4 | 0.05–20 μm | 6.49 nm | [154] |
| Xylan-derived N-CQDs without N-doping | 2 | ||||||||||
| Hematin | Fluorescent static quenching (IFE) | Solvothermal | p-aminobenzoic acid (PABA) | 180 °C, 12 h | n/a | Silica column chromatography | 11.8 | n/a | 0.5–10 μm | 0.25 μm | [155] |
| glutathione | CQDs-H2O2- TMB (3, 3′, 5, 5′-tetramethylbenzidine) system | Acid refux | wood soot HNO3 | 140 °C, 12 h | n/a | Dialysis (MWCO n/a, 2 days) Centrifugation (16,000× g rpm, 15 min) Ultra-filtration | 2.3 | n/a | 0.05–20 μm | 0.016 μm | [156] |
| Pesticides: propanyl parathion dimethoate chlorpyrifos pyrimicarb | FRET | Hydrothermal | riboflavin | 160 °C (1, 2, 5 h) 180 °C (1, 2, 5 h) 200 °C (1, 2, 5 h) | n/a | Dialysis (12 h/12 h) of “CD6 (180 °C, 5 h)” | 3.47 ± 0.02 (CD6) | n/a | n/a | n/a | [157] |
| H2O2 | M-CQDs-H2O2-TMB (3, 3′, 5, 5′-tetramethylbenzidine) system | Hydrothermal | Mustard seeds | 180 °C, 4 h | n/a | Centrifugation (15,000× g rpm, 20 min) | 4.58 ± 0.26 | 4.6 | 0.02−0.20 mm | 0.015 mm | [158] |
| ascorbic acid | 10−70 µm | 3.26 µm | |||||||||
| Acetone | enhancement in PL emission | Hydrothermal | citric acid borax p-phenylenediamine | 180 °C, 5 h | N, B-doping | Filtration (0.22 μm) Dialysis (500 Da, 24 h) | 3.53 | n/a | 1–200 μm | 0.54 μm | [143] |
| Dopamine | static quenching mechanism | 0.1–70 μm | 11 nm | ||||||||
| m-benzenediol (resorcinol) | Fluorescence enhancing | Hydrothermal | Cryptococcus | 160 ℃, 1 h | n/a | Centrifugation (5000× g rpm, 5 min) Filtration (0.22 mm) Dialysis (100 Da, 12 h) | 4–9 | 14.13 | 2 × 10−8–4 × 10−4 m | 8.68 nm | [159] |
| p-benzenediol ((hydroquinone) | Fluorescence quenching | 4 × 10−9–2 × 10−5 M | 6.7 nm | ||||||||
| Cu (II) | Fluorescence quenching | Dry-Pyrolysis | Finger millet ragi (Eleusine coracana) | 300 °C (step of 5 °C/min), 3 h at 300 °C | n/a | Filtration (0.22 µm) | 6 | n/a | 0–100 μm | 10 nm | [160] |
| Ellagic acid | Fluorescent static quenching (inner filter) | Hydrothermal | citric acid diethylenetriamine | 180 °C, 10 h | N-doping | Dialysis tube (500 Da, time n/a) | 3.16 | 84.79 | 0.01–50 μm | 0.01 μm | [161] |
| Dimethoate | fluorescence static quenching | Dry-Pyrolysis | pork rib bones from food waste | 1st) 700 °C, 5 h 2nd) Centrifugation (6000× g rpm, 6 min) 3rd) 200 °C, 10 h | dithizone | Filtration (0.22 μm) | 4.2 ± 1.2 | n/a | 0.15 μm–5.0 μm | 0.064 μm | [162] |
| curcumin in dietary foods | IFE and dynamic interaction | Solvothermal | m-phenylenediamine citric acid | 180 °C, 12 h | N-doping | Dialysis (500–1000 Da, 24/24/24 h) | 4.5 | 61.7 | 0.01–25.0 μm | 28.7 nm | [163] |
| Diazinon | Fluorescent static quenching | Hydrothermal (Aqueous extracts) | Blue rose petals | 200 °C, 2 h | n/a | Filtration | 37 | 46 | 0.02–10 µm | 0.01 µm | [164] |
| Red rose petals | 39 | 44 | |||||||||
| Yellow rose petals | 33 | 48 | |||||||||
| Solvothermal (Alcoholic extracts) | Blue rose petals | 30 | 43 | ||||||||
| Red rose petals | 27 | 46 | |||||||||
| Yellow rose petals | 26 | 47 | |||||||||
| Ovalbumin (OVA) | C-MIPs@FITC | Hydrothermal | citric acid ethylenediamine | 200 °C, 5 h. | N-doping | Dialysis (MWCO n/a, 24 h) | n/a | n/a | 0.05–2 μm | 15.4 nm | [165] |
| SiO2 NPs (i) OVA (i) APTES (i) TEOS (ii) NH3.H2O (ii) FITC (iii) | (i) Stirring 1 h (ii) Stirring 24 h (iii) Stirring 2 h | (MIPs@FITC) OVA fluorescent imprinted nanoparticles | (ii) centrifugation and washing w/methanol/acetic acid (9:1, v/v) (iii) washing w/ethanol | 90 | |||||||
| CDs MIPs@FITC PBS | mixing | (C-MIPs@FITC) molecularly imprinted ratiometric fluorescence nanosensor | n/a | n/a |
| (A) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Application | CCT (K) | Lmax (cd m−2) | ηc (cd A−1) | CQDs Synthesis Method | Precursors | Reaction Conditions | Modifications and Functionalization | Purification | Average Size (nm) | QY (%) | Ref. |
| LEDs | n/a | 1882–4762 | 1.22–5.11 | Solvothermal | Phloroglucinol (PG) | 200 °C, 2 h, 5 h, 9 h, and 24 h | n/a | Silica gel column chromatography (Dichloromethane and Methanol (variable) | 1.9–3.9 | blue CQDs: 66 green CQDs: 72 yellow CQDs: 62 red CQDs: 54 | [166] |
| LEDs | 6000 | n/a | n/a | Reflux | Diammonium hydrogen citrate Urea | 180 °C, 15 min | N-doping | Centrifugation (6000× g rpm, 20 min) | 4–10 | 16 | [167] |
| LEDs | n/a | 2–174 | 9 × 10−4–2 × 10−3 | Hydrothermal or Hot injection | Citric acid HDA/urea/EDA/PDA | 200 °C, 5 h or 160 °C, 16 h | N-doping HDA/EDA/PDA hybridization | Centrifugation (4500× g rpm, Time n./a) | 3.6, 5.4, 3.3, 31.7 | EDA-CQDs: 43 PDA-CQDs: 8 HAD-CQDs: 40 Urea-CQDs: 35 | [168] |
| LEDs | 2520 | n/a | n/a | Hydrothermal | 2,7-dihydroxynaphthalene Ethylenediamine | 180 °C, 12 h | N-doping | Centrifugation (8000× g rpm, 5 min) | 3.31 | 62.98 | [169] |
| LEDs | n/a | n/a | n/a | Microwave-assisted | Citric acid o-Phenylene-diamine (oPD) | 125 °C, 5 min | N-doping | Dialysis (1000 Da, 30 min) | 1.1 | 5.4 | [38] |
| LEDs | 6250 | n/a | n/a | Reflux | Citric acid Tris-HMA | 225 °C, 20 min | N-doping Polymer passivation | Dialysis (1000 Da, 2 days) | 3.35 | 23 | [92] |
| LEDs | 3913, 5994, 10000 | n/a | n/a | Solvothermal | o-Phenylenediamine 4-aminobenzenesulfonic acid folic acid boric acid acetic acid terephthalic acid tartaric acid | 180 °C, 12 h | N-doping | Dialysis (MWCO n/a, Time n/a) in ethanol | 3.01 | b-CQDs: 25 c-CQDs: 36 g-CQDs: 28 yg-CQDs: 72 y-CQDs: 45 o-CQDs: 59 r-CQDs: 47 dr-CQDs: 52 w-CQDs: 39 | [170] |
| (B) | |||||||||||
| Application | Jsc (mA/cm2) | Voc (V) | η (%) | CQDs Synthesis Method | Precursors | Reaction Conditions | Modifications and Functionalization | Purification | Average Size (nm) | QY (%) | Ref. |
| Solar converters | 22.2 | 1.05 | 15 | Hydrothermal | Citric acid Urea | 200 °C, 6 h | TiO2 | Wash with deionized water and ethanol numerous times | 30–40 | n/a | [171] |
| Solar converters | n/a | n/a | n/a | Hydrothermal | Citric acid Ethylenediamine TRIS | 200 °C, 6 h | N-doping | Dialysis (3000 Da, 2 h) | 2 | 50 | [172] |
| Hot-injection | PbBr2/PbCl2 salts | - | n/a | - | 11–14 | CsPb (Br0.8Cl0.2)3 QDs: 70 CsPb(Br0.2I0.8)3 QDs: 60 | |||||
| Solar converters | 12.67–18.49 | 0.46–0.48 | 3.26–4.97 | Microwave-assisted | Citric acid Urea | 800 W, 5 min | N-doping | Filtration with a cylinder filtration membrane filter (0.22 μm) | 2.9 | 80 | [173] |
| Solar converters | 35.98 | 0.62 | 17.86 | Hydrothermal | Citric acid KH-792 Thiourea | 180 °C, 12 h | N-doping | Wash with hexane, 3 times Dialysis (MWCO n/a, 24 h) | 2–3 | 20.68 | [174] |
| Solar converters | 17.4 | 0.738 | 9.04 | Hydrothermal | Lotus root powder Sulphuric acid Ammonium hydroxide | 170 °C, 6 h | S- or N-doping | Dialysis (3500 Da, Time n/a) | 4–5 | n/a | [175] |
| Solar converters | 1.60 | 0.54 | 1.20 | Solvothermal | Jaggery syrup Urea | 140 °C, 2.5 h | N-doping | Centrifugation (12,000× g rpm, 30 min) | 9.5 | 9.8 | [176] |
| Solar converters | 4.5 | 0.495 | 1.6 | Hydrothermal | Phloroglucinol | 200 °C, 9 h + 70 °C, 30 min | n/a | Dialysis (1000 Da, 12 h) | 1.74 | 40 | [177] |
| Target/ Contaminant | Photocatalysis Mechanism | CQDs Synthesis Method | Modifications and Functionalization | Precursors | Reaction Conditions | Purification | QY (%) | Average Size (nm) | Degradation (%) | Degradation Time (min) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodamine B Methylene blue | Oxidation by formation of holes and superoxide radical anions | Microwave assisted | N-doping | Citric acid 1,2-Phenylenediamine | 700 W, 7 min | Filtration and SEC | 39.2 | 2 | 70 | 150 | [114] |
| Acid Blue | Formation of reactive oxide species (ROS) | Hydrothermal | N-doping | Grass | 180 °C, 2 h | Wash with distilled water | n/a | <10 | 100 (for all) | 30 | [178] |
| Acid Red | 30 | ||||||||||
| Eosin Y | 90 | ||||||||||
| Eriochrome Black T | Immediate decomposition no radiation required | ||||||||||
| Methyl orange | Immediate decomposition no radiation required | ||||||||||
| Methylene blue | 90 | ||||||||||
| Rhodamine B Methylene Blue | Oxidation by formation of holes and active oxygen/hydroxyl radicals | Hydrothermal | n/a | Palm powder | 200 °C, 7 h | Filtration with membrane filter (0.22 μm) Dialysis (500 Da membrane, 48 h) | 0.9 | 3.54 | 71.7 and 94.2, respectively | 45 (both) | [179] |
| Methyl viologen | photoinduced electron transfer | Hydrothermal (for amorphous synthesis (a-)) | n/a | Citric acid (Cit) | 180 °C, 24 h | - | 1.0 a-Cit-CDs | 9–12 | 3.45 × 10−8 M s−1 | n/a | [180] |
| Glucose (Glu) | 200 °C, 24 h | Centrifugation (n/a time) and Filtration | 1.8 a-Glu-CDs | 0.65 × 10−8 M s−1 | |||||||
| Fructose (Fru) | 200 °C, 24 h | 0.3 a-Fru-CDs | 4.37 × 10−8 M s−1 | ||||||||
| Pyrolytic (for graphitic synthesis (g-)) | Citric acid (Cit) | 220 °C, 48 h | Dialysis (1000 Da membrane, 24 h) | 1.2 g-Cit-CDs | 7–9 | 5.06 × 10−8 M s−1 | |||||
| Glucose (Glu) | 220 °C, 48 h | 2.3 g-Glu-CDs | 2–7 | 1.07 × 10−8 M s−1 | |||||||
| Fructose (Fru) | 220 °C, 48 h | 0.7 g-Fru-CDs | 0.5 μm–1.5 μm | 0.67 × 10−8 M s−1 | |||||||
| Tetracycline Bisphenol A Rhodamine B | Direct hole oxidation reaction | Hydrothermal | n/a | n/a | n/a | n/a | 80 | 5 | 60 73 91.8 | 120 150 20 | [181] |
| Rose bengal | Formation of singlet oxygen | Bottom-up condensation | P-doping | Pluronic F-68 | n/a | n/a | 31 | <21 | 86 | 180 | [182] |
| CO2 | Reduction by accumulated electrons under the assistance of H+ | Microwave-assisted | N, S-doping | Citric acid Thiourea | 800 W, 7 min | Dialysis (MWCO n/a, 24 h) | n/a | 3.5 | n/a | 360 | [183] |
| Tetracycline | Oxidation by formation of holes and superoxide radical anions | Hydrothermal | N-doping | Ammonium citrate Ethylenediamine | 200 °C, 5 h | Dialysis (1000 Da, 24 h) | n/a | 8 | 97 | 25 | [184] |
| Crystal violet dye | Oxidation by formation of holes and photo-generated electrons | Hydrothermal and mixed-calcination | n/a | Ascorbic acid Glycol | 160 °C, 70 min | n/a | n/a | 4–9 | 70 | 300 | [185] |
| Amoxicillin | Oxidation by formation of holes and hydroxyl radicals | Hydrothermal and calcination | potassium titanate (K2Ti6O13) | n/a | n/a | n/a | 80 | n/a | 100 | 90 | [186] |
| Sulfamethazine | Oxidation by formation of holes, superoxide radical anions and photo-generated electrons | Calcination | N-doping | Citric acid Urea | 550 °C, 3 h | n/a | n/a | 2–5 | 97.3 | 50 | [187] |
| Indigo carmine | Oxidation by formation of holes and photo-generated electrons | Solvothermal | N-doping | Aniline | 180 °C, 10 h | Centrifugation (10 000× g rpm, 5 min) Dialysis (3500 Da, 24 h) | 0.25 | 2.0 | 97 | 120 | [188] |
| 2-aminonaphthalene | 0.11 | 2.2 | |||||||||
| 2-anthracylamine | 0.28 | 2.6 | |||||||||
| 1-aminopyrene | 0.14 | 2.2 | |||||||||
| Benzene p-xylene Toluene | CQDs/TiO2 nanocomposites improved interfacial charge transfer; increased light absorption; narrower bandgap | Pyrolysis | CQDs/TiO2 nanocomposites | Citric acid | 180 ℃, 40 h | n/a | n/a | 2.4 | 31 64 99 | 140 | [189] |
| Application | Target | Targeting Ligands | Receptor/Target | CQDs Synthesis Method | Precursors | Reaction Conditions | Modifications and Functionalization | Purification | Average Size (nm) | QY (%) | Ref. |
| Antimicrobial | Escherichia coli (E. coli) Staphylococcus aureus (S. aureus) | n/a | n/a | Pyrolysis | Aloe-Vera extract | 190 °C, 20 min | n/a | Dialysis (300 Da, Time n/a) | 6–8 | 12.3 | [51] |
| Bacteria identification | Escherichia coli (E. coli) Desulfovibrio desulfuricans (D. desulfuricans) Staphylococcus sciuri (S. sciuri) Listeria monocytogenes (L. monocytogenes) Staphylococcus aureus (S. aureus) Pseudomonas aeruginosa (P. aeruginosa) | 3-Aminophenylboronic acid vancomycin hydrochloride polymyxin B sulfate | cis-diol (D)-Ala-(D)-Ala peptide LPS | Hydrothermal | Ammonium citrate dibasic 3-Aminophenylboronic acid vancomycin hydrochloride polymyxin B sulfate | 180 °C, 4 h | 3-Aminophenylboronic acid vancomycin hydrochloride polymyxin B sulfate hybridized | Centrifugation (10,000× g rpm, 15 min) Dialysis (1000 Da, overnight) | 3–6 | n/a | [118] |
| Antimicrobial | Escherichia coli Bacillus subtilis | n/a | n/a | Hydrothermal | Polyvinylpyrrolidone | 200 °C, 6 h | N-doping | Filtration with a cylinder filtration membrane filter (0.22 μm) Centrifugation (8000× g rpm, 15 min) | 6.5 | 6 | [190] |
| Poly (sodium- 4styrene sulfonate) | S-doping | 5 | 9.5 | ||||||||
| Antimicrobial | E. coli S. aureus AREC KREC | n/a | n/a | Smoking | Cigarette | Cigarette smoke of 200 cigarettes was dissolved into 1 L deionized water | n/a | Dialysis (1000 Da, Time n/a) | 5.4 | n/a | [191] |
| Antimicrobial | S. aureus Bacillus subtilis Bacillus sp. WL-6 E. coli ampicillin-resistant E. coli Rhizoctonia Solani Pyricularia Grisea | n/a | Bacterial and fungal DNA/RNA | Electrochemical | Vitamin C | 0.1 A direct current (DC) power, 3 weeks | n/a | Dialysis (500 Da, Time n/a) | 5 | 30 | [192] |
| Antiviral | HCoV229E | Boronic acid | S-receptor | Hydrothermal | Citric acid Ethylenediamine | Temperature: n/a, 5 h | Boronic acid functionalization (Click chemistry) | Centrifugation Dialysis (MWCO n/a, 24 h) | 4.5 | 40 | [193] |
| Antiviral | Japanese encephalitis Zika Dengue Porcine parvovirus Adenovirus-associated virus | n/a | n/a | Hydrothermal | Benzoxazine monomers (BZM) | 180 °C, 12 h | n/a | Centrifugation (10,000× g rpm, 10 min) Dialysis (MWCO n/a, Time n/a) | 4.4 | 13.1 | [194] |
| Antiviral | Porcine reproductive Respiratory syndrome virus (PRRSV) | Glycyrrhizic acid | n/a | Hydrothermal | Glycyrrhizic acid | 180 °C, 7 h | n/a | Centrifugation (10,000× g rpm, 10 min) Dialysis (14,000 Da, 8 h) | 11.4 | 1.41 | [195] |
| Antiviral | White spot syndrome virus (WSSV) | n/a | n/a | n/a | Polyamine | n/a | n/a | n/a | n/a | n/a | [196] |
| Antimicrobial | Bacillus subtilis | n/a | n/a | Reflux in concentrated nitric acid | Carbon nano-powders | Temp (°C) n/a, 48 h | EDA: EDA-CDots | Dialysis (500 Da, 48 h) Centrifugation (1000× g, time n/a) | 4–5 | 20 | [81] |
| EPA: EPA-CDots | 4–5 | 20 | |||||||||
| PEI1200: PEI1200-CDots | 4–6 | 12 | |||||||||
| PEI600: PEI600-CDots | n/a | n/a | |||||||||
| Hydrothermal | Citric acid PEI1200 | n/a | PEI1200/CA-CDots-1 PEI1200/CA-CDots-2 PEI1200/CA-CDots-3 | n/a | 10 | 60 | |||||
| Antimicrobial | Fusarium oxysporum S. aureus P. aeruginosa B. subtilis E. coli MCF-7 HepG2 cells | n/a | n/a | Microwave-assisted | Pomegranate extract Watermelon extract PEG 200 | Microwave radiation for 2 min at the interval of 10 s each | Polymer passivation | n/a | 1–5 | n/a | [131] |
| Antibacterial | Staphyloccocus aureus Escherichia coli | Polyurethane (PU) | n/a | Swell encapsulation-shrink method (hCQDss/PU) | Pluronic F-68 | ambient temperature, 48 h | n/a | n/a | n/a | n/a | [182] |
| Antiviral | avian leukosis virus subgroup J (ALV-J) | gp85 protein | n/a | Hydrothermal | Humic acid polytetrafluoroethylene | 180 °C, 5 h | gp85 protein | Centrifugation (3000× g rpm, 15 min) Dialysis (1000 Da, 48 h) Centrifugation (12,000× g rpm, 15 min) | 3–5 | n/a | [197] |
| Antiviral | enterovirus 71 (EV71) | curcumin | ROS generation PGE2 production translation of EV71- and EV71-induced eIF4G cleavage phosphorylated p38 kinase | Dry-carbonization in muffle furnace | Curcumin | 120 °C, 2 h (Cur-CQDs-120) | n/a | Centrifugation (35,000× g, 1 h) Dialysis in sodium chloride solution (0.5–1 kDa, 5 h) Dialysis in ultrapure water solution (0.5–1 kDa, 18 h) | 4.2 | <0.1 | [198] |
| 150 °C, 2 h (Cur-CQDs-150) | 4.5 | ||||||||||
| 180 °C, 2 h (Cur-CQDs-180) | 4.8 | ||||||||||
| 210 °C, 2 h (Cur-CQDs-210) | 5.2 | ||||||||||
| Antimicrobial | methicillin-resistant Staphylococcus aureus (MRSA) Ampicillin-resistant Escherichia coli | n/a | Negative charge of bacterial membranes DNA | Hydrothermal | bis-quaternary ammonium salt (BQAS) | 200 °C, 12 h | N-doping | Dialysis (500 Da, 24 h) | 2.15 | n/a | [199] |
| Antimicrobial | methicillin-sensitive Staphylococcus aureus (ATCC 6538) methicillin-resistant Staphylococcus aureus (ATCC 4300) Escherichia coli (ATCC 25922) Staphylococcus epidermidis (ATCC 49134) Bacillus cereus (ATCC 14579) | n/a | n/a | Hydrothermal | polyethyleneimine citric acid | 250 ºC, 4 h | N-doping | Dialysis (≥12,000 Da, 24 h) | 70.2, 32.2, 11.5 | PEI:CA ratio (1:0.5) –31 (1:1)–53 (1:2)–7.6 | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, H.B.A.; Martins, C.S.M.; Prior, J.A.V. You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials 2021, 11, 611. https://doi.org/10.3390/nano11030611
Sousa HBA, Martins CSM, Prior JAV. You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials. 2021; 11(3):611. https://doi.org/10.3390/nano11030611
Chicago/Turabian StyleSousa, Helena B. A., Catarina S. M. Martins, and João A. V. Prior. 2021. "You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots" Nanomaterials 11, no. 3: 611. https://doi.org/10.3390/nano11030611
APA StyleSousa, H. B. A., Martins, C. S. M., & Prior, J. A. V. (2021). You Don’t Learn That in School: An Updated Practical Guide to Carbon Quantum Dots. Nanomaterials, 11(3), 611. https://doi.org/10.3390/nano11030611