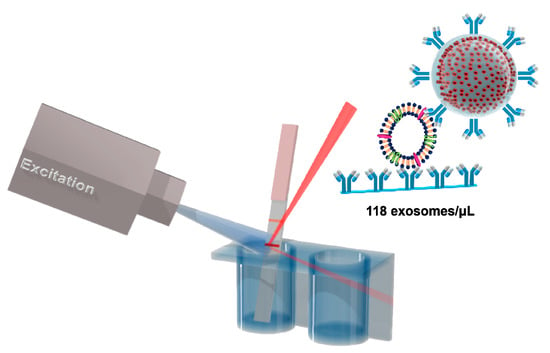
Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Synthesis of Silica Coated Multi-Quantum Dot (M–QD–SNs)
2.3. M–QD–SNs–Antibody Conjugates
2.4. Test Strip Preparation
2.5. Fluorescence Assay Procedure
2.6. Human Foreskin Fibroblast (HFF) Exosome Extraction
2.7. Control Liposome Preparation
2.8. Exosome Immunoblotting
2.9. Characterization
3. Results
3.1. Synthesis and Characterization of M–QD–SNs
3.2. Characterization of Extracted HFF Exosomes and Conjugation of CD63 Ab to M–QD–SNs
3.3. Detection of HFF Exosomes at Various Concentration with M-QD-SNs for Fluorescent Lateral Flow Assay (fLFA)
3.4. Identification of HFF Exosome Detection Specificity by M-QD-SNs in fLFA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosaka, N.; Kogure, A.; Yamamoto, T.; Urabe, F.; Usuba, W.; Prieto-Vila, M.; Ochiya, T. Exploiting the message from cancer: The diagnostic value of extracellular vesicles for clinical applications. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Deng, C.-X. Current Progresses of Exosomes as Cancer Diagnostic and Prognostic Biomarkers. Int. J. Biol. Sci. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in cancer diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Khodashenas, S.; Khalili, S.; Moghadam, M.F. A cell ELISA based method for exosome detection in diagnostic and therapeutic applications. Biol. Lett. 2019, 41, 523–531. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Hikita, T.; Miyata, M.; Watanabe, R.; Oneyama, C. Sensitive and rapid quantification of exosomes by fusing luciferase to exosome marker proteins. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalalian, S.H.; Ramezani, M.; Jalalian, S.A.; Abnous, K.; Taghdisi, S.M. Exosomes, new biomarkers in early cancer detection. Anal. Biochem. 2019, 571, 1–13. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Natl. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.-P.; Zhong, X.-Q.; Hua, B.; Liu, M.-Y.; Jing, F.-X.; Lou, X.-H.; Yao, S.-H.; Xiang, J.-Q.; Jin, Q.-H.; Zhao, J.-L. Nano-ELISA for highly sensitive protein detection. Biosens. Bioelectron. 2009, 24, 2836–2841. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, K.-D.; Huang, C.; Wen, T.; Jiao, Y.-J.; Zhu, J.; Zhang, Q.; Ding, S.-N. Rapid detection of Shiga toxin type II using lateral flow immunochromatography test strips of colorimetry and fluorimetry. Analyst 2020, 145, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Beswick, A.D.; Kunutsor, S.K.; Wilson, M.J.; Whitehouse, M.R.; Blom, A.W. The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis. JBJS 2016, 98, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- Li, S.; Jin, Q.; Jiang, X.; Park, J.J.J.H. Frontier and Future Development of Information Technology in Medicine and Education: ITME 2013; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 269. [Google Scholar]
- Yang, H.; Li, D.; He, R.; Guo, Q.; Wang, K.; Zhang, X.; Huang, P.; Cui, D. A novel quantum dots–based point of care test for syphilis. Nanoscale Res. Lett. 2010, 5, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays–from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lakowicz, J.R. Silver-enhanced fluorescence emission of single quantum dot nanocomposites. Chem. Commun. 2009, 3, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, G.; Wu, H.; Lin, Y. Quantum-Dot-based electrochemical immunoassay for high-throughput screening of the prostatespecific antigen. Small 2008, 4, 82–86. [Google Scholar] [CrossRef]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H. Ultrasensitive, Biocompatible, Quantum-Dot-Embedded Silica Nanoparticles for Bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Ha, Y.; Jung, H.S.; Jeong, S.; Kim, H.M.; Kim, T.H.; Cha, M.G.; Kang, E.J.; Pham, X.H.; Jeong, D.H.; Jun, B.H. Fabrication of Remarkably Bright QD Densely-Embedded Silica Nanoparticle. Bull. Korean Chem. Soc. 2019, 40, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Goftman, V.V.; Markin, A.V.; De Saeger, S.; Goryacheva, I.Y. Multicolored silica coated CdSe core/shell quantum dots. In Proceedings of the Saratov Fall Meeting 2015: Third International Symposium on Optics and Biophotonics and Seventh Finnish-Russian Photonics and Laser Symposium (PALS), Saratov, Russia, 21 April 2016; p. 991716. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Jo, A.; Kim, T.H.; Kim, D.-M.; Kim, H.-M.; Seong, B.; Kim, J.; Pham, X.-H.; Jung, H.S.; Lee, S.H.; Jeong, D.H. Sensitive detection of virus with broad dynamic range based on highly bright quantum dot-embedded nanoprobe and magnetic beads. J. Ind. Eng. Chem. 2020, 90, 319–326. [Google Scholar] [CrossRef]
- Cho, J.; Jung, Y.K.; Lee, J.-K.; Jung, H.-S. Highly efficient blue-emitting CdSe-derived core/shell gradient alloy quantum dots with improved photoluminescent quantum yield and enhanced photostability. Langmuir 2017, 33, 3711–3719. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lee, J.-H.; Kang, H.-D.; Han, C.-Y.; Bae, S.M.; Lee, Y.; Hwang, J.Y.; Yang, H. Highly fluorescence-stable blue CdZnS/ZnS quantum dots against degradable environmental conditions. J. Alloys Compd. 2014, 610, 511–516. [Google Scholar] [CrossRef]
- Jun, S.; Jang, E. Bright and stable alloy core/multishell quantum dots. Angew. Chem. Int. Ed. 2013, 52, 679–682. [Google Scholar] [CrossRef]
- Wolcott, A.; Gerion, D.; Visconte, M.; Sun, J.; Schwartzberg, A.; Chen, S.; Zhang, J.Z. Silica-coated CdTe quantum dots functionalized with thiols for bioconjugation to IgG proteins. J. Phys. Chem. B 2006, 110, 5779–5789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; López-Cobo, S.; Reyburn, H.T.; Costa-García, A.; López-Martín, S.; Yáñez-Mó, M.; Cernuda-Morollón, E.; Paschen, A.; Valés-Gómez, M.; Blanco-López, M.C. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J. Extracell. Vesicles 2016, 5, 31803–31812. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Cao, Y.; Huang, Y.; Xu, L.-P.; Zhang, X.; Wang, S. Enhanced lateral flow assay with double conjugates for the detection of exosomes. Sci. China Chem. 2018, 61, 1423–1429. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Shi, Q.; Du, D.; Liu, D.; Luo, Y.; Xu, W.; Lin, Y. Au@Pd nanopopcorn and aptamer nanoflower assisted lateral flow strip for thermal detection of exosomes. Anal. Chem. 2019, 91, 13986–13993. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| No. | Signal-Generating Reagent | LOD (exosomes/µL) | Reference |
|---|---|---|---|
| 1 | Gold nanoparticles | 8.54 × 105 | [31] |
| 2 | Double gold–nanoparticle conjugates | 1.3 × 103 | [32] |
| 3 | Au@Pd nanopopcorn | 1.4 × 104 | [33] |
| 4 | M–QD–SNs | 0.2 × 102 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Oh, C.; An, J.; Baek, S.; Bock, S.; Kim, J.; Jung, H.-S.; Song, H.; Kim, J.-W.; Jo, A.; et al. Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials 2021, 11, 768. https://doi.org/10.3390/nano11030768
Kim H-M, Oh C, An J, Baek S, Bock S, Kim J, Jung H-S, Song H, Kim J-W, Jo A, et al. Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials. 2021; 11(3):768. https://doi.org/10.3390/nano11030768
Chicago/Turabian StyleKim, Hyung-Mo, Chiwoo Oh, Jaehyun An, Seungki Baek, Sungje Bock, Jaehi Kim, Heung-Su Jung, Hobeom Song, Jung-Won Kim, Ahla Jo, and et al. 2021. "Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection" Nanomaterials 11, no. 3: 768. https://doi.org/10.3390/nano11030768
APA StyleKim, H. -M., Oh, C., An, J., Baek, S., Bock, S., Kim, J., Jung, H. -S., Song, H., Kim, J. -W., Jo, A., Kim, D. -E., Rho, W. -Y., Jang, J. -Y., Cheon, G. J., Im, H. -J., & Jun, B. -H. (2021). Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials, 11(3), 768. https://doi.org/10.3390/nano11030768