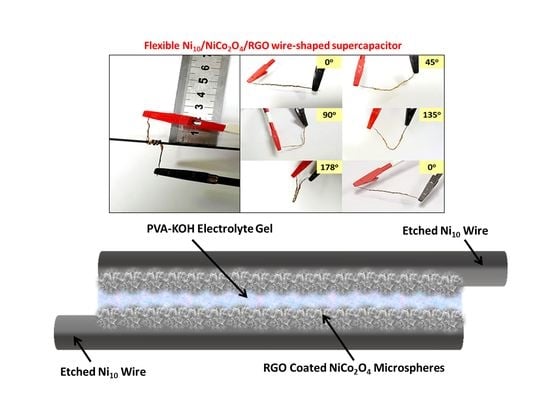
NiCo2O4/RGO Hybrid Nanostructures on Surface-Modified Ni Core for Flexible Wire-Shaped Supercapacitor
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Wire Electrodes
2.2. Fabrication of Wire-Shaped Symmetric Supercapacitor
2.3. Materials Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Structural, Surface-Morphological and Chemical Compositional Analysis
3.2. The Electrochemical Characteristics of Ni/NCO/RGO Wire Electrodes
3.3. The Electrochemical Characteristics of Ni10/NCO/RGO Wire-Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, A.-Y.; Chang, C.-C.; Lai, Y.-W.; Chen, P.-R.; Xu, B.-C. Improving the Supercapacitor Performance by Dispersing SiO2 Microspheres in Electrodes. ACS Omega 2020, 5, 11522–11528. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Hsiao, C.-H.; Su, T.-Y.; Hsieh, P.-Y.; Chen, Y.-L.; Chueh, Y.-L.; Lee, C.-Y.; Tai, N.-H. Bioinspired networks consisting of spongy carbon wrapped by graphene sheath for flexible transparent supercapacitors. Commun. Chem. 2019, 2, 1–10. [Google Scholar] [CrossRef]
- Chu, Y.; Xiong, S.; Li, B.; Qian, Y.; Xi, B. Designed Formation of MnO2@NiO/NiMoO4Nanowires@Nanosheets Hierarchical Structures with Enhanced Pseudocapacitive Properties. ChemElectroChem 2016, 3, 1347–1353. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Liu, J.; Liu, C.; Xu, J.; Jiang, Q.; Jia, Y.; Jiang, F.; Duan, X.; Liu, P. Fabrication of PEDOT:PSS/rGO fibers with high flexibility and electrochemical performance for supercapacitors. Electrochim. Acta 2021, 365, 137363. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Dirican, M.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Carbon black-based porous sub-micron carbon fibers for flexible supercapacitors. Appl. Surf. Sci. 2021, 537, 147914. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Lin, H.; Chen, Y. Flexible fiber-shaped supercapacitors with high energy density based on self-twisted graphene fibers. J. Power Sources 2019, 433, 226711. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Z.; Gao, C. Graphene fiber based supercapacitors: Strategies and perspective toward high performances. J. Energy Chem. 2018, 27, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xie, L.; Wen, Z.; Chen, C.; Chen, X.; Wei, A.; Cheng, P.; Xie, X.; Sun, X. Coaxial Triboelectric Nanogenerator and Supercapacitor Fiber-Based Self-Charging Power Fabric. ACS Appl. Mater. Interfaces 2018, 10, 42356–42362. [Google Scholar] [CrossRef]
- Cho, S.; Patil, B.; Yu, S.; Ahn, S.; Hwang, J.; Park, C.; Do, K.; Ahn, H. Flexible, Swiss roll, fiber-shaped, asymmetric supercapacitor using MnO2 and Fe2O3 on carbon fibers. Electrochim. Acta 2018, 269, 499–508. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCo2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2013, 1, 2468–2473. [Google Scholar] [CrossRef]
- Sun, D.S.; Li, Y.H.; Wang, Z.Y.; Cheng, X.P.; Jaffer, S.; Zhang, Y.F. Understanding the mechanism of hydrogenated NiCo2O4 nanograss supported on Ni foam for enhanced-performance supercapacitors. J. Mater. Chem. A 2016, 4, 5198–5204. [Google Scholar] [CrossRef]
- Xu, S.; Yang, D.; Zhang, F.; Liu, J.; Guo, A.; Hou, F. Fabrication of NiCo2O4 and carbon nanotube nanocomposite films as a high-performance flexible electrode of supercapacitors. RSC Adv. 2015, 5, 74032–74039. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.; Yang, L.; Zhu, Y.; Liu, P.; Xing, O.; Zhou, J.; Wang, M.; Luo, H.; et al. A Low-Cost, Self-Standing NiCo2 O4 @CNT/CNT Multilayer Electrode for Flexible Asymmetric Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Chang, L.; Li, C.; Ouyang, H.; Huang, J.; Huang, Q.; Xu, Z. Flexible NiCo2O4@carbon/carbon nanofiber electrodes fabricated by a combined electrospray/electrospinning technique for supercapacitors. Mater. Lett. 2019, 240, 21–24. [Google Scholar] [CrossRef]
- Chen, J.; Ma, T.; Chen, M.; Peng, Z.; Feng, Z.; Pan, C.; Zou, H.; Yang, W.; Chen, S. Porous NiCo2O4@Ppy core-shell nanowire arrays covered on carbon cloth for flexible all-solid-state hybrid supercapacitors. J. Energy Storage 2020, 32, 101895. [Google Scholar] [CrossRef]
- Peng, M.; Tian, X.; Li, D.; Wang, Q.; Zhang, D. Flexible high-energy asymmetric supercapacitors based on PANI@CNT-graphene and NiCo2O4@N-C electrode. Mater. Lett. 2020, 272, 127859. [Google Scholar] [CrossRef]
- Urhan, B.K. Demir, Ümit Electrochemical fabrication of Ni or Ni(OH)2@Ni nanoparticle-decorated reduced graphene oxide for supercapacitor applications. Electrochim. Acta 2019, 302, 109–118. [Google Scholar] [CrossRef]
- Zhou, D.; Niu, H.; Lin, H.; Yang, X.; Jiang, H.; Zhang, T.; Wang, Q.; Qu, F. 3D interconnected networks of a ternary hierarchical carbon nanofiber/MnO2/Ni(OH)2 architecture as integrated electrodes for all-solid-state supercapacitors. RSC Adv. 2016, 6, 71882–71892. [Google Scholar] [CrossRef]
- Nong, J.; Lan, G.; Jin, W.; Luo, P.; Guo, C.; Tang, X.; Zang, Z.; Wei, W. Eco-friendly and high-performance photoelectrochemical anode based on AgInS2 quantum dots embedded in 3D graphene nanowalls. J. Mater. Chem. C 2019, 7, 9830–9839. [Google Scholar] [CrossRef]
- Vimuna, V.; Athira, A.; Babu, K.D.; Xavier, T. Simultaneous stirring and microwave assisted synthesis of nanoflakes MnO2/rGO composite electrode material for symmetric supercapacitor with enhanced electrochemical performance. Diam. Relat. Mater. 2020, 110, 108129. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; Zhou, L.; Zhang, R.; Zhang, C.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@layered double hydroxide core–shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, J.; Wu, J.; Yang, Y.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Hierarchical nanothorns MnCo2O4 grown on porous/dense Ni bi-layers coated Cu wire current collectors for high performance flexible solid-state fiber supercapacitors. J. Power Sources 2018, 393, 54–61. [Google Scholar] [CrossRef]
- Ramadoss, A.; Kang, K.-N.; Ahn, H.-J.; Kim, S.-I.; Ryu, S.-T.; Jang, J.-H. Realization of high performance flexible wire supercapacitors based on 3-dimensional NiCo 2 O 4 /Ni fibers. J. Mater. Chem. A 2016, 4, 4718–4727. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, B.; Jayaseelan, S.S.; Seo, M.-K.; Kim, H.-Y.; Kim, B.-S. NiCo2S4 nanosheet-decorated 3D, porous Ni film@Ni wire electrode materials for all solid-state asymmetric supercapacitor applications. Nanoscale 2017, 9, 18819–18834. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Naderi, L.; Mohammadi, R. High-Performance Fiber-Shaped Flexible Asymmetric Microsupercapacitor Based on Ni(OH)2 Nanoparticles-Decorated Porous Dendritic Ni–Cu Film/Cu Wire and Reduced Graphene Oxide/Carbon Fiber Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 14574–14588. [Google Scholar] [CrossRef]
- Naderi, L.; Shahrokhian, S. Nickel molybdate nanorods supported on three-dimensional, porous nickel film coated on copper wire as an advanced binder-free electrode for flexible wire-type asymmetric micro-supercapacitors with enhanced electrochemical performances. J. Colloid Interface Sci. 2019, 542, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, F.; Zhu, B.; Guo, L.; Yang, Y.; Hao, R.; Wang, H.; Liu, Y.; Wang, W.; Guo, X.; et al. Flexible Integrated Electrical Cables Based on Biocomposites for Synchronous Energy Transmission and Storage. Adv. Funct. Mater. 2016, 26, 3472–3479. [Google Scholar] [CrossRef]
- Sun, P.; Lin, R.; Wang, Z.; Qiu, M.; Chai, Z.; Zhang, B.; Meng, H.; Tan, S.; Zhao, C.; Mai, W. Rational design of carbon shell endows TiN@C nanotube based fiber supercapacitors with significantly enhanced mechanical stability and electrochemical performance. Nano Energy 2017, 31, 432–440. [Google Scholar] [CrossRef]
- Palasantzas, G.; De Hosson, J.T.M. Influence of surface roughness on the adhesion of elastic films. Phys. Rev. E 2003, 67, 021604. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Saito, R. Effect of surface roughening of aluminum plates on the strength of bonds formed between aluminum and polyphenylene sulfide by thermosonic bonding. IOP Conf. Series Mater. Sci. Eng. 2014, 61. [Google Scholar] [CrossRef] [Green Version]
- Bodner, T.; Behrendt, A.; Prax, E.; Wiesbrock, F. Correlation of surface roughness and surface energy of silicon-based materials with their priming reactivity. Mon. Chem. Chem. Mon. 2012, 143, 717–722. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [Green Version]
- Agudosi, E.S.; Abdullah, E.C.; Numan, A.; Mubarak, N.M.; Aid, S.R.; Benages-Vilau, R.; Gómez-Romero, P.; Khalid, M.; Omar, N. Fabrication of 3D binder-free graphene NiO electrode for highly stable supercapattery. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, J.; Pan, Z.; Zhang, J.; Zhao, J.; Wang, X.; Zhang, C.; Yao, Y.; Lu, W.; Li, Q.; et al. Stretchable fiber-shaped asymmetric supercapacitors with ultrahigh energy density. Nano Energy 2017, 39, 219–228. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, G.; Zhang, H.; Li, S. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Stolyarova, S.; Saridakis, E.; Chayen, N.E.; Nemirovsky, Y. A Model for Enhanced Nucleation of Protein Crystals on a Fractal Porous Substrate. Biophys. J. 2006, 91, 3857–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, C.; Pang, J.; Lu, Q.; Liu, T. Effects of surface topography of material on nucleation site density of dropwise condensation. Chem. Eng. Sci. 2008, 63, 874–880. [Google Scholar] [CrossRef]
- Stolyarova, S.; Baskin, E.; Chayen, N.E.; Nemirovsky, Y. Possible model of protein nucleation and crystallization on porous silicon. Physica Status Solidi 2005, 202, 1462–1466. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yao, X.-Z.; Luo, T.; Jia, Y.; Liu, J.-H.; Huang, X.-J. Facile Synthesis of Urchin-like NiCo2O4 Hollow Microspheres with Enhanced Electrochemical Properties in Energy and Environmentally Related Applications. ACS Appl. Mater. Interfaces 2014, 6, 3689–3695. [Google Scholar] [CrossRef]
- Da, Y.; Liu, J.; Zhou, L.; Zhu, X.; Chen, X.; Fu, L. Engineering 2D Architectures toward High-Performance Micro-Supercapacitors. Adv. Mater. 2019, 31, e1802793. [Google Scholar] [CrossRef] [Green Version]
- Abouelamaiem, D.I.; He, G.; Parkin, I.P.; Neville, T.P.; Jorge, A.B.; Ji, S.; Wang, R.; Titirici, M.-M.; Shearing, P.R.; Brett, D.J.L. Synergistic relationship between the three-dimensional nanostructure and electrochemical performance in biocarbon supercapacitor electrode materials. Sustain. Energy Fuels 2018, 2, 772–785. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Gao, J.; Shi, X.; Chang, J.; Dong, Y.; Zheng, S.; Wang, X.; Feng, L.; Wu, Z. Hierarchical Ordered Dual-Mesoporous Polypyrrole/Graphene Nanosheets as Bi-Functional Active Materials for High-Performance Planar Integrated System of Micro-Supercapacitor and Gas Sensor. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Zhang, H.; Passerini, S.; Qian, X. Two-Dimensional Titanium Carbide/RGO Composite for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15661–15667. [Google Scholar] [CrossRef]
- Singh, A.; Chandra, A. Enhancing Specific Energy and Power in Asymmetric Supercapacitors—A Synergetic Strategy based on the Use of Redox Additive Electrolytes. Sci. Rep. 2016, 6, 25793. [Google Scholar] [CrossRef] [Green Version]
- Mary, A.J.C.; Sathish, C.R.I.; Vinu, A.; Bose, A.C. Electrochemical Performance of rGO/NiCo2O4@ZnCo2O4 Ternary Composite Material and the Fabrication of an all-Solid-State Supercapacitor Device. Energy Fuels 2020, 34, 10131–10141. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Kim, T.; Choi, W.; Shin, H.-C.; Choi, J.-Y.; Kim, J.M.; Park, M.-S.; Yoon, W.-S. Applications of Voltammetry in Lithium Ion Battery Research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, S.; Nagamuthu, S.; Ryu, K.-S. CuCo 2 O 4 flowers/Ni-foam architecture as a battery type positive electrode for high performance hybrid supercapacitor applications. Electrochim. Acta 2017, 238, 99–106. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, F.; Yan, Q.; Wu, X. Investigation on electrochemical behaviors of NiCo2O4 battery-type supercapacitor electrodes: The role of an aqueous electrolyte. Inorg. Chem. Front. 2017, 4, 1642–1648. [Google Scholar] [CrossRef]
- Li, C.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Hierarchical Zn-Co-S Nanowires as Advanced Electrodes for All Solid State Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1702014. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Fouad, H.; Cho, M.H.; Ansari, S.G. Intercalated reduced graphene oxide and its content effect on the supercapacitance performance of the three dimensional flower-like β-Ni(OH)2 architecture. New J. Chem. 2017, 41, 10467–10475. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. NiO-CNT composite for high performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Sadhukhan, P.; Saha, S.; Das, S. Morphological behaviour, electronic bond formation and electrochemical performance study of V2O5-polyaniline composite and its application in asymmetric supercapacitor. Mater. Res. Bull. 2018, 107, 379–390. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Stoller, M.D.; Zhu, Y.; Ji, H.; Murali, S.; Wu, Y.; Perales, S.; Clevenger, B.; Ruoff, R.S. Highly Conductive and Porous Activated Reduced Graphene Oxide Films for High-Power Supercapacitors. Nano Lett. 2012, 12, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, G. Preparation of Highly Conductive Graphene Hydrogels for Fabricating Supercapacitors with High Rate Capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Shi, B.; Huang, F.; Rong, F.; Que, R. Three-dimensional NiCo2O4@NiCo2O4 core–shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Zhang, J.; Shewale, P.S.; Yun, K.-S. Yun Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire. Energies 2019, 12, 3127. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.-N.; Ramadoss, A.; Min, J.-W.; Yoon, J.-C.; Lee, D.; Kang, S.J.; Jang, J.-H. Wire-Shaped 3D-Hybrid Supercapacitors as Substitutes for Batteries. Nano-Micro Lett. 2020, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, A.; Arvand, M.; Daneshvar, S. A novel flexible wire-shaped supercapacitor with enhanced electrochemical performance based on hierarchical Co(OH)2@Ni(OH)2 decorated porous dendritic Ni film/Ni wire. J. Alloy. Compd. 2021, 856, 158101. [Google Scholar] [CrossRef]
- Lee, H.; Lee, G.; Yun, J.; Keum, K.; Hong, S.Y.; Song, C.; Kim, J.W.; Lee, J.H.; Oh, S.Y.; Kim, D.S.; et al. Facile fabrication of a fully biodegradable and stretchable serpentine-shaped wire supercapacitor. Chem. Eng. J. 2019, 366, 62–71. [Google Scholar] [CrossRef]
- Sun, H.; You, X.; Deng, J.; Chen, X.; Yang, Z.; Ren, J.; Peng, H. Novel Graphene/Carbon Nanotube Composite Fibers for Efficient Wire-Shaped Miniature Energy Devices. Adv. Mater. 2014, 26, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, H.; Liu, X.Y.; Zhu, S.J.; Jia, J.Q.; Xu, C.H.; Dong, F.; Wen, Z.Q.; Zhang, Y.X. Low-cost high-performance asymmetric supercapacitors based on Co2AlO4@MnO2 nanosheets and Fe3O4 nanoflakes. J. Mater. Chem. A 2016, 4, 2096–2104. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Lin, J.; Sun, X.; Mao, S. The effect of various electrolyte cations on electrochemical performance of polypyrrole/RGO based supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 28666–28673. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, P.; Zhang, H.; Zhang, D.; Sun, X.; Ma, Y. Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type MnO2 nanosheets for supercapacitor applications. Electrochim. Acta 2013, 89, 523–529. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Churikov, A.V.; Ivanishcheva, I.A.; Ushakov, A.V. Lithium diffusion in Li3V2(PO4)3-based electrodes: A joint analysis of electrochemical impedance, cyclic voltammetry, pulse chronoamperometry, and chronopotentiometry data. Ionics 2016, 22, 483–501. [Google Scholar] [CrossRef]
- Churikov, A.V.; Pridatko, K.I.; Ivanishchev, A.V.; Ivanishcheva, I.A.; Gamayunova, I.M.; Zapsis, K.V.; Sycheva, V.O. Impedance spectroscopy of lithium-tin film electrodes. Russ. J. Electrochem. 2008, 44, 550–557. [Google Scholar] [CrossRef]















| Element | Value |
|---|---|
| Rs (Ω) | 295 |
| C1 (μF) | 2944 |
| R1 (kΩ) | 128.9 |
| C2 (μF) | 9.325 |
| R2 (Ω) | 19.01 |
| W (mS∙s1/2) | 3.333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shewale, P.S.; Yun, K.-S. NiCo2O4/RGO Hybrid Nanostructures on Surface-Modified Ni Core for Flexible Wire-Shaped Supercapacitor. Nanomaterials 2021, 11, 852. https://doi.org/10.3390/nano11040852
Shewale PS, Yun K-S. NiCo2O4/RGO Hybrid Nanostructures on Surface-Modified Ni Core for Flexible Wire-Shaped Supercapacitor. Nanomaterials. 2021; 11(4):852. https://doi.org/10.3390/nano11040852
Chicago/Turabian StyleShewale, Prashant Shivaji, and Kwang-Seok Yun. 2021. "NiCo2O4/RGO Hybrid Nanostructures on Surface-Modified Ni Core for Flexible Wire-Shaped Supercapacitor" Nanomaterials 11, no. 4: 852. https://doi.org/10.3390/nano11040852
APA StyleShewale, P. S., & Yun, K. -S. (2021). NiCo2O4/RGO Hybrid Nanostructures on Surface-Modified Ni Core for Flexible Wire-Shaped Supercapacitor. Nanomaterials, 11(4), 852. https://doi.org/10.3390/nano11040852