Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Al Nanopowder
2.2. Consolidation of Al Nanopowder
2.3. Characterization
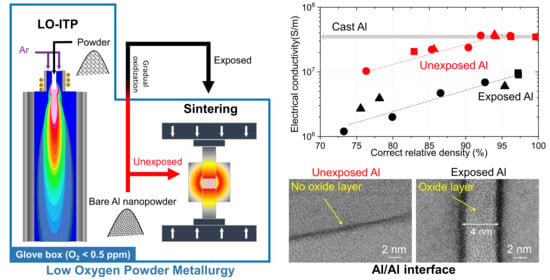
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.W.; Lian, J.; Wang, J.; Cai, X.Q.; Wang, Y.; Wang, D.P.; Wang, Z.M.; Liu, Y.C. Diffusion bonding of Ni3Al-based alloy using a Ni interlayer. J. Alloys Compd. 2020, 819, 153324. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Liu, Y.; Wang, Z. Direct diffusion bonding of immiscible tungsten and copper at temperature close to copper’s melting point. Mater. Des. 2018, 1137, 473–480. [Google Scholar] [CrossRef]
- Soyal, T.; Kou, S.; Tat, D.; Pasang, T. Macrosegregation in dissimilar-metal fusion welding. Acta Mater. 2016, 110, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; You, G.; Yuan, G.; Zhang, X. Microstructure and mechanical properties of dissimilar inertia friction welding of 7A04 aluminum alloy to AZ31 magnesium alloy. J. Alloys Compd. 2017, 695, 3267–3277. [Google Scholar] [CrossRef]
- Yuan, R.; Deng, S.; Cui, H.; Chen, Y.; Lu, F. Interface characterization and mechanical properties of dual beam laser welding-brazing Al/steel dissimilar metals. J. Manuf. Process. 2019, 40, 37–45. [Google Scholar] [CrossRef]
- Kim, T.H.; Howlader, M.M.R.; Itoh, T.; Suga, T. Room temperature Cu–Cu direct bonding using surface activated bonding method. J. Vac. Sci. Technol. A 2003, 21, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Tong, Q.-Y. Room temperature metal direct bonding. Appl. Phys. Lett. 2006, 89, 1–3. [Google Scholar] [CrossRef]
- Hinterreiter, A.P.; Rebban, G.; Flotgen, C. Surface pretreated low-temperature aluminum-aluminum wafer bonding. Microstyst. Technol. 2018, 24, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Matsumae, T.; Kurashima, Y.; Takagi, H. Surface activated bonding of Ti/Au and Ti/Pt/Au films after vacuum annealing for MEMS packaging. Microelectron. Eng. 2018, 197, 76–82. [Google Scholar] [CrossRef]
- Yang, L.; Hosoda, N.; Suga, T. TEM investigation of the stainless steel/aluminum interface created by the surface activated bonding method. Nucl. Instrum. Methods Phys. Res. B 1997, 121, 519–523. [Google Scholar] [CrossRef]
- Tian, W.; Chen, F.; Cheng, R.; Li, Z.; Pang, G. Corrosion Properties of Pure Aluminum Prepared by Spark Plasma Sintering (SPS) Using Different Grain Size of Aluminium Powders as Raw Material. Int. J. Electrochem. Sci. 2020, 15, 9120–9134. [Google Scholar] [CrossRef]
- Demirskyi, D.; Agrawai, D.; Ragulya, A. Neck growth kinetics during microwave sintering of copper. Scr. Mater. 2010, 62, 552–555. [Google Scholar] [CrossRef]
- Li, F.; Pan, J. Modelling “Nano-Effects” in Sintering; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Jeong, S.; Lee, S.H.; Jo, Y.; Lee, S.S.; Seo, Y.-H.; Ahn, B.W.; Kim, G.; Jang, G.-E.; Park, J.-U.; Ryu, B.-H.; et al. Air-stable, surface-oxide free Cu nanoparticles for highly conductive Cu ink and their application to printed graphene transistors. J. Mater. Chem. C 2013, 1, 2704–2710. [Google Scholar] [CrossRef] [Green Version]
- Janardhanan, R.; Karuppaiah, M.; Hebalkar, N.; Rao, T.N. Syntesis and surface chemistry of nano silver particles. Polyhedron 2009, 28, 2522–2530. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Ji, H.; Li, M. Highly Conductive Cu–Cu Joint Formation by Low-Temperature Sintering of Formic Acid-Treated Cu Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar] [CrossRef]
- Nagae, T.; Yokota, M.; Nose, M.; Tomida, S.; Kamiya, T.; Saji, S. Effects of pulse current on an aluminum powder oxide layer during pulse current pressure sintering. Mater. Trans. 2002, 43, 1390–1397. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Park, D.H.; Kawasaki, A.; Park, Y.; Silvain, J.F.; Park, Y. Spark Plasma Sintering Behavior of Pure Aluminum Depending on Various Sintering Temperatures. Met. Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.K.; Yang, M.T.; Desai, R.A.; Yu, X.; Liu, Z.; Chen, C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7, 733–736. [Google Scholar] [CrossRef]
- Jiang, J.; Bao, B.; Li, M.; Sun, J.; Zhang, C.; Li, Y.; Li, F.; Yao, X.; Song, Y. Fabrication of Transparent Multilayer Circuits by Inkjet Printing. Adv. Mater. 2015, 28, 1420–1426. [Google Scholar] [CrossRef]
- Baek, S.H.; Song, H.W.; Lee, S.; Kim, J.-E.; Kim, Y.H.; Wi, J.-S.; Ok, J.G.; Park, J.S.; Hong, S.; Kwak, M.K.; et al. Gold Nanoparticle-Enhanced and Roll-to-Roll Nanoimprinted LSPR Platform for Detecting Interleukin-10. Front. Chem. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Menumerov, E.; Golze, S.D.; Hughes, R.A.; Neretina, S. Arrays of highly complex noble metal nanostructures using nanoimprint lithography in combination with liquid-phase epitaxy. Nanoscale 2018, 10, 18186–18194. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ramaprabhu, S. Synthesis and Thermal Conductivity of Copper Nanoparticle Decorated Multiwalled Carbon Nanotubes Based Nanofluids. J. Phys. Chem. C 2008, 112, 9315–9319. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Synthesis of silver nanoparticle decorated multiwalled carbon nanotubes-graphene mixture and its heat transfer studies in nanofluid. AIP Adv. 2013, 3, 012111. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, Y.; Suzuki, K.; Yamaguchi, W.; Takagi, K. Cold welding behavior of fine bare aluminum powders prepared by new low oxygen induction thermal plamsa system. J. Alloys Compd. 2018, 768, 608–612. [Google Scholar] [CrossRef]
- Rasche, S.; Kuna, M. Improved small punch testing and parameter identification of ductile to brittle materials. Int. J. Pres. Ves. Pip. 2015, 125, 23–34. [Google Scholar] [CrossRef]
- Stegmann, H.; Ritz, T.; Utezz, D.; Hubner, R.; Zschech, E. Sample preparation for atomic-resolution STEM at low voltages by FIB. Ultramicroscopy 2009, 114, 62–71. [Google Scholar]
- Evertsson, J.; Bertram, F.; Zhang, F.; Rullik, L.; Merte, L.R.; Shipilin, M.; Soldemo, M.; Ahmadi, S.; Vinogradov, N.; Carla, F.; et al. The thickness of native oxides on aluminum alloys and single crystals. Appl. Surf. Sci. 2015, 349, 826–832. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film. J. Appl. Phys. 1963, 34, 1793. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.Q.; Ma, W.G. Barrier parameter variation in Al-Al2O3-metal tunnel junctions. Appl. Phys. Lett. 1992, 61, 2542–2544. [Google Scholar] [CrossRef]
- Holmqvist, T.; Meschke, M.; Pekola, J.P. Double oxidation scheme for tunnel junction fabrication. J. Vac. Sci. Technol. B 2008, 26, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Kato, M. Hall-Petch relationship and dislocation model for deformation of ultrafine-grained and nanocrystalline metals. Mater. Trans. 2014, 55, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Matsui, I.; Ono, S.; Takigawa, Y.; Uesugi, T.; Higashhi, K. Fabrication of bulk nanocrystalline Al electrodeposited from a dimethysulfone bath. Mater. Sci. Eng. A 2012, 550, 363–366. [Google Scholar] [CrossRef]
- Hayes, R.W.; Witkin, D.; Zhou, F.; Lavernia, E.J. Deformation and activation volume of cryomilled ultrafine-grained aluminum. Acta Mater. 2004, 52, 4259–4271. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, X.; Lloyd, D.J.; Hansen, N. Microstructure and strength of commercial purity aluminum (AA1200) cold-rolled to large strains. Acta Mater. 2002, 50, 3789–3802. [Google Scholar] [CrossRef]
- Lv, S.; Jia, C.; He, X.; Wan, Z.; Li, X.; Qu, X. Superplastic Deformation and Dynamic Recrystallization of a Novel Disc Superalloy GH4151. Materials 2019, 12, 3667. [Google Scholar] [CrossRef] [Green Version]
- Kanikawa, N.; Huang, X.; Tsuji, N.; Hansen, N. Strengthening mechanisms in nanostructured high-purity aluminum deformed to high strain and annealed. Acta Mater. 2009, 57, 4198–4208. [Google Scholar] [CrossRef]
- Li, S.X.; Cui, G.R. Dependence of strength, elongation, and toughness on grain size in metallic structural materials. J. Appl. Phys. 2007, 101, 083525. [Google Scholar] [CrossRef]
- Bruchhausen, M.; Holmstrom, S.; Simonovski, I.; Austin, T.; Lapetite, J.-M.; Ripplinger, S.; de Haan, F. Recent development in small punch testing: Tensile properties and DBTT. Thoeor. Appl. Fract. Mec. 2016, 86, 2–10. [Google Scholar] [CrossRef]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Saravanan, M.; Pillai, R.M.; Pai, B.C.; Brahmakumar, M.; Ravi, K.R. Equal channel angular pressing of pure aluminum-an analysis. Bull. Mater. Sci. 2006, 29, 679–684. [Google Scholar]
- Kawasaki, M.; Lee, H.-J.; Ahn, B.; Zhilyaev, A.P.; Langdon, T.G. Evolution of hardness in ultrafine-grained metals processed by high-pressure torsion. J. Mater. Res. Technol. 2014, 3, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziem, W.; Hamada, A.S.; Hassan, M.A. Effect of the Cyclic Extrusion and Compression Processing on Microstructure and Mechanical Properties of Al-1%Cu Alloy. Key Eng. Mater. 2018, 780, 93–97. [Google Scholar] [CrossRef]
- Yu, H.; Lu, C.; Tieu, K.; Kong, C. Fabrication of nanostructured aluminum sheets using four-layer accumulative roll bonding. Mater. Manuf. Process. 2014, 29, 448–453. [Google Scholar] [CrossRef]
- Shigeta, M.; Cheng, Y.; Choi, S.; Watanabe, T. Formation mechanism of titanium boride nanoparticles by RF induction thermal plasma. Chem. Eng. Trans. 2012, 183, 483–491. [Google Scholar]
- Zhang, X.; Hayashida, R.; Tanaka, M.; Watanabe, T. Synthesis of carbon-coated silicon nanoparticles by induction thermal plasma for lithium ion battery. Powder Technol. 2020, 371, 26–36. [Google Scholar] [CrossRef]
- Aktekin, B.; Cakmak, G.; Oztyrk, T. Induction thermal plasma synthesis of Mg2Ni nanoparticles. Int. J. Hydrog. Energ. 2014, 39, 9859–9864. [Google Scholar] [CrossRef]
- Pant, A.; Seth, T.; Raut, V.B.; Gahbhiye, V.P.; Newale, S.P.; Nandi, A.K.; Prasanth, H.; Pandey, R.K. Preparation of Nano Aluminium Powder (NAP) using a Thermal Plasma: Process Development and Characterization. Cent. Eur. Energ. Mater. 2016, 13, 53–71. [Google Scholar] [CrossRef]
- Bissett, H.; Walt, I.J.; Havenga, J.L.; Nel, J.T. Titanium and zirconium metal powder spheroidization by thermal plasma processes. J. S. Afr. Inst. Min. Metall. 2015, 115, 937–942. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Hirayama, Y.; Liu, Z.; Kwon, H.; Kobashi, M.; Takagi, K. Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique. Nanomaterials 2021, 11, 1182. https://doi.org/10.3390/nano11051182
Kim D, Hirayama Y, Liu Z, Kwon H, Kobashi M, Takagi K. Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique. Nanomaterials. 2021; 11(5):1182. https://doi.org/10.3390/nano11051182
Chicago/Turabian StyleKim, Dasom, Yusuke Hirayama, Zheng Liu, Hansang Kwon, Makoto Kobashi, and Kenta Takagi. 2021. "Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique" Nanomaterials 11, no. 5: 1182. https://doi.org/10.3390/nano11051182
APA StyleKim, D., Hirayama, Y., Liu, Z., Kwon, H., Kobashi, M., & Takagi, K. (2021). Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique. Nanomaterials, 11(5), 1182. https://doi.org/10.3390/nano11051182