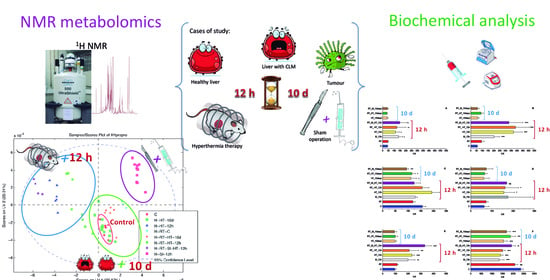
Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Induction and Intra-Arterial Infusion
2.2. Thermal Therapy
2.3. Blood and Tissue Collection
2.4. Biochemical Analysis
2.5. Metabolomic Studies
2.6. Statistical Analyses for Serum Samples
3. Results
3.1. Biochemical Changes
3.2. Metabolomic Changes in Hepatic Tissue
3.3. Metabolomic Changes of Tumor Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Experimental Group | ALT (IU/l) | AST (IU/l) | AP (IU/l) | Amylase (IU/l) | CK (IU/l) | LDH (IU/l) | Creatinine (mg/dl) |
|---|---|---|---|---|---|---|---|
| C | 42 ± 4.6 | 57 ± 3.61 | 158 ± 16.5 | 2361 ± 152 | 98.3 ± 14.2 | 63.7 ± 27.6 | 0.413 ± 0.035 |
| RT | 46 ± 4 | 94.3 ± 21.9 | 119 ± 8.7 | 2083 ± 140 | 115 ± 8.5 | 72.7 ± 9.3 | 0.397 ± 0.038 |
| SI_12h | 128 ± 38 | 202 ± 45.8 | 128 ± 8.7 | 1349 ± 231 | 180 ± 66.1 | 208 ± 118 | 0.384 ± 0.057 |
| HT_12h | 106 ± 34 | 370 ± 73.9 | 194 ± 30.4 | 1410 ± 114 | 191 ± 51.2 | 172 ± 42.7 | 0.454 ± 0.057 |
| RT_HT_12h | 124 ± 77 | 388 ± 119 | 156 ± 26 | 1503 ± 121 | 129 ± 96.4 | 179 ± 58.9 | 0.462 ± 0,091 |
| RT_SI_HT_12h | 129 ± 73 | 331 ± 121 | 124 ± 24.3 | 1928 ± 180 | 265 ± 82.7 | 170 ± 1.53 | 0.462 ± 0.135 |
| HT_10d | 41 ± 7 | 61 ± 11.4 | 111 ± 21.9 | 1554 ± 181 | 98.4 ± 11.4 | 61.7 ± 26.1 | 0.385 ± 0.054 |
| RT_HT_10d | 38 ± 5.2 | 89.7 ± 20 | 115 ± 14 | 2303 ± 227 | 100 ± 18.8 | 66.2 ± 11.4 | 0.328 ± 0.053 |
| RT_SI_10d | 42 ± 6.6 | 58.7 ± 14.6 | 95 ± 7.6 | 1692 ± 184 | 93.8 ± 17.2 | 73.8 ± 30.4 | 0.408 ± 0.043 |
| Metabolite | ppm | C | H_RT_C | T |
|---|---|---|---|---|
| leucine/isoleucine | 0.966 | 1.00 | 0.57 | 1.26 |
| valine | 0.984 | 1.00 | 0.12 | 1.53 |
| D-hydroxybutyrate | 1.187 | 1.00 | 2.23 | 0.30 |
| lactate | 1.325 | 1.00 | 0.85 | 1.29 |
| alanine | 1.492 | 1.00 | 0.99 | 1.09 |
| lysine | 1.719 | 1.00 | 0.46 | 1.34 |
| acetate, acetoacetate | 1.921 | 1.00 | 2.36 | 0.22 |
| N-acetyl functions of glycoprotein | 2.053 | 1.00 | 0.31 | 1.43 |
| glutamine | 2.152 | 1.00 | 0.73 | 1.15 |
| glutamate | 2.359 | 1.00 | 0.37 | 1.40 |
| pyruvate | 2.408 | 1.00 | 0.24 | 1.39 |
| gluthathione (oxidized) | 2.559 | 1.00 | 1.46 | 0.77 |
| aspartate | 2.805 | 1.00 | 0.46 | 1.34 |
| creatine | 3.040 | 1.00 | 0.43 | 1.52 |
| choline | 3.225 | 1.00 | 0.17 | 1.39 |
| GPC (sn-glycerol-3-phosphocholine) | 3.233 | 1.00 | 0.52 | 0.77 |
| PC (phosphatidylcholine) | 3.250 | 1.00 | 1.38 | 0.40 |
| taurine, trimethylamine-N-oxide | 3.271 | 1.00 | 2.04 | 0.13 |
| betaine | 3.359 | 1.00 | 2.51 | 1.34 |
| 𝜶-glucose | 3.424 | 1.00 | 1.18 | 1.43 |
| glycine | 3.553 | 1.00 | 0.59 | 1.43 |
| citrate | 3.562 | 1.00 | 0.20 | 1.43 |
| threonine | 3.609 | 1.00 | 0.22 | 1.46 |
| free glycerol | 3.651 | 1.00 | 0.17 | 1.49 |
| ascorbic acid | 4.504 | 1.00 | 2.42 | 0.20 |
| Metabolite | ppm | C | H_RT_C | H_HT_12h | H_RT_HT_12h | H_RT_SI_HT_12h | H_SI_12h | H_HT_10d | H_RT_HT_10d |
|---|---|---|---|---|---|---|---|---|---|
| leucine/isoleucine | 0.966 | 1.00 | 0.30 | 0.46 | 0.34 | 0.50 | 0.81 | 1.18 | 0.47 |
| valine | 0.984 | 1.00 | 0.19 | 0.23 | 0.17 | 0.28 | 0.72 | 1.20 | 0.38 |
| D-hydroxybutyrate | 1.187 | 1.00 | 2.24 | 11.39 | 6.54 | 10.84 | 5.39 | 0.95 | 2.62 |
| lactate | 1.325 | 1.00 | 0.81 | 3.24 | 1.94 | 3.12 | 1.86 | 1.23 | 0.98 |
| alanine | 1.492 | 1.00 | 1.60 | 1.39 | 1.36 | 1.36 | 1.15 | 0.92 | 1.26 |
| lysine | 1.719 | 1.00 | 0.20 | 0.21 | 0.17 | 0.27 | 0.71 | 1.20 | 0.39 |
| acetate, acetoacetate | 1.921 | 1.00 | 1.47 | 0.22 | 0.87 | 0.24 | 0.59 | 0.84 | 1.27 |
| glutamate | 2.133 | 1.00 | 0.41 | 1.22 | 0.68 | 1.23 | 1.14 | 1.21 | 0.52 |
| pyruvate | 2.408 | 1.00 | 0.26 | 0.49 | 0.33 | 0.53 | 0.83 | 1.19 | 0.44 |
| glutamine | 2.480 | 1.00 | 0.21 | 1.43 | 0.74 | 1.42 | 1.23 | 1.23 | 0.48 |
| gluthathione (oxidized) | 2.565 | 1.00 | 0.91 | 3.91 | 2.06 | 3.77 | 2.24 | 1.17 | 1.04 |
| asparagine | 2.960 | 1.00 | 0.46 | 2.86 | 1.53 | 2.78 | 1.84 | 1.21 | 0.75 |
| creatine | 3.040 | 1.00 | 0.43 | 0.87 | 0.60 | 0.89 | 0.98 | 1.16 | 0.59 |
| choline | 3.225 | 1.00 | 2.75 | 9.24 | 5.83 | 8.78 | 4.43 | 0.84 | 2.82 |
| GPC (sn-glycerol-3-3-phosphocholine) | 3.233 | 1.00 | 0.37 | 0.55 | 0.42 | 0.59 | 0.85 | 1.17 | 0.52 |
| PC (phosphatidylcholine) | 3.250 | 1.00 | 1.14 | 3.53 | 1.85 | 1.63 | 2.02 | 3.41 | 2.10 |
| betaine | 3.359 | 1.00 | 2.25 | 4.65 | 3.41 | 4.44 | 2.49 | 0.76 | 2.12 |
| 𝜶-glucose | 3.424 | 1.00 | 3.07 | 4.07 | 2.84 | 3.87 | 1.93 | 0.43 | 2.45 |
| glycine | 3.553 | 1.00 | 1.35 | 6.01 | 3.26 | 5.75 | 3.04 | 1.07 | 1.22 |
| citrate | 3.562 | 1.00 | 0.53 | 0.25 | 0.31 | 0.30 | 0.73 | 1.22 | 0.53 |
| threonine | 3.609 | 1.00 | 1.01 | 0.57 | 0.81 | 0.59 | 0.81 | 1.00 | 0.98 |
| free glycerol | 3.651 | 1.00 | 1.77 | 13.50 | 7.19 | 12.83 | 6.07 | 1.12 | 2.29 |
| glucose-1-phosphate-(glycogen) | 4.504 | 1.00 | 0.00 | 0.22 | 0.07 | 0.28 | 0.73 | 1.25 | 0.26 |
| ascorbic acid | 4.515 | 1.00 | 0.26 | 0.92 | 0.48 | 0.94 | 1.02 | 1.23 | 0.41 |
| glycogen | 5.416 | 1.00 | 0.01 | 0.15 | 0.03 | 0.22 | 0.70 | 1.25 | 0.25 |
| Metabolite | ppm | T_C | T_HT_10d | T_HT_12h | T_SI_HT_12h |
|---|---|---|---|---|---|
| leucine/isoleucine | 0.966 | 1.00 | 0.28 | 2.76 | 0.21 |
| valine | 0.984 | 1.00 | 0.28 | 0.15 | 2.57 |
| D-hydroxybutyrate | 1.187 | 1.00 | 2.39 | 0.43 | 0.17 |
| lactate | 1.325 | 1.00 | 2.04 | 0.75 | 0.32 |
| alanine | 1.492 | 1.00 | 0.43 | 3.45 | 0.31 |
| lysine | 1.719 | 1.00 | 0.52 | 2.14 | 0.23 |
| acetate, acetoacetate | 1.921 | 1.00 | 0.46 | 2.41 | 0.51 |
| N-acetyl functions of glycoprotein | 2.053 | 1.00 | 0.20 | 2.14 | 0.85 |
| pyruvate | 2.408 | 1.00 | 0.18 | 1.22 | 2.51 |
| gluthathione (oxidized) | 2.559 | 1.00 | 0.27 | 1.11 | 1.83 |
| aspartate | 2.692 | 1.00 | 0.22 | 2.24 | 0.77 |
| dimethylglycine | 2.941 | 1.00 | 0.61 | 1.38 | 1.15 |
| anserine | 2.962 | 1.00 | 0.67 | 0.89 | 1.43 |
| creatine | 3.042 | 1.00 | 1.58 | 1.66 | 0.24 |
| choline | 3.225 | 1.00 | 1.04 | 1.94 | 0.14 |
| GPC (sn-glycerol-3-phosphocholine) | 3.233 | 1.00 | 0.12 | 2.40 | 0.62 |
| taurine, trimethylamine-N-oxide | 3.271 | 1.00 | 0.23 | 2.55 | 0.99 |
| glycine | 3.555 | 1.00 | 0.65 | 1.71 | 1.61 |
| betaine | 3.359 | 1.00 | 1.90 | 0.16 | 0.19 |
| 𝜶-glucose | 3.424 | 1.00 | 0.32 | 0.48 | 8.09 |
| citrate | 3.562 | 1.00 | 0.92 | 1.95 | 0.31 |
References
- Turcotte, S.; Jarnagin, W.R. Liver & Portal Venous System. In Current Diagnosis and Treatment Surgery; Doherty, G.M., Ed.; McGraw-Hill Education: New York, NY, USA, 2015; pp. 557–559. [Google Scholar]
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; International Agency for Research on Cancer: Lyon, France, 2020; ISBN 9789283204299. [Google Scholar]
- Cugat, E.; Mir-Labrador, J.; Cortese, S.; Pareja, E.; Fuster, J.; Santoyo, J. Cirugía hepática. In Cirugía Hepática; Campos, R.R., Paricio, P.P., Eds.; Aran: Madrid, Spain, 2018; pp. 34–44. ISBN 9788417554125. [Google Scholar]
- García Pérez, R. Estudio Comparativo de Regeneración Hepática Inducida por Técnica ALPPS versus Ligadura Portal en un Modelo Experimental en Rata; University of Murcia: Murcia, Spain, 2005; Volume 281. [Google Scholar]
- Van den Eynde, M.; Hendlisz, A. Treatment of Colorectal Liver Metastases: A Review. Rev. Recent Clin. Trials 2009, 4, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Goldstein, L.S.; Dewhirst, M.W.; Repacholi, M.; Kheifets, L. Summary, conclusions and recommendations: Adverse temperature levels in the human body. Int. J. Hyperth. 2003, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Gillams, A.R. Liver ablation therapy. Br. J. Radiol. 2004, 77, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Karino, T.; Koga, S.; Maeta, M. Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Jpn. J. Surg. 1988, 18, 276–283. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor magnetic hyperthermia induced by RGD-functionalized Fe3O4 nanoparticles, in an experimental model of colorectal liver metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Arriortua, O.K. Funcionalización y Estudio de Nanopartículas de Magnetita para su Aplicación en Terapias de Hipertermia Magnética; Universidad del País Vasco: Biscay, Spain, 2015. [Google Scholar]
- Echevarria-Uraga, J.J.; García-Alonso, I.; Plazaola, F.; Insausti, M.; Etxebarria, N.; Saiz-López, A.; Fernández-Ruanova, B. Study of the intra-arterial distribution of Fe3O4 nanoparticles in a model of colorectal neoplasm induced in rat liver by MRI and spectrometry. Int. J. Nanomed. 2012, 7, 2399–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echevarria-Uraga, J.J.; García-Alonso Montoya, I.; Miguélez Vidales, J.L.; Sanz Sánchez, F.; Plazaola Muguruza, F.; Insausti Peña, M.; Etxebarria Loizate, N.; Fernández-Ruanova, B. Magnetic resonance imaging and spectrometric study of the distribution of thermotherapeutic magnetofluid after intra-arterial administration in an experimental model of liver metastases. Radiologia 2012, 54, 251–259. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Irazola Duñabeitia, M.; Carrero, J.A.; Etxebarria Loizate, N.; García-Alonso, I.; Echevarria-Uraga, J.J. Intra-Arterial Infusion of Magnetic Nanoparticle-Based Theragnostic Agent to Treat Colorectal Cancer Liver Implants in Rats. Eur. Surg. Res. 2020. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; García-Alonso, I.; Garaio, E.; Insausti, M.; Aizpurua, J.M.; Etxebarria-Loizate, N.; Saiz-Lopez, A.; Echevarria-Uraga, J.J. RGD-Magnetic-Nanoparticles induced hyperthermia was followed by necrosis of colorectal cancer cells growing in the rat liver. In Proceedings of the 50th Congress of the European Society for Surgical Research, Liverpool, UK, 10–13 June 2015; Volume 55, p. 14. [Google Scholar]
- García-Alonso, I.; Echevarria-Uraga, J.J.; Marín, H.; Herrero de la Parte, B.; Plazaola, F. Perfusion of Fe3O4 Magnetic Nanoparticles into Experimental Liver Metastases. In Proceedings of the 2012 European Society for Surgical Research Congress, Lille, France, 6–9 June 2012; Volume 50, p. 200. [Google Scholar]
- Herrero de la Parte, B.; García-Alonso, I.; Mar-Medina, C.; Iturrizaga, S.; Saiz-López, A.; Hernández-Farto, L.; Del Campo-Clemente, C.; Echevarría-Uraga, J.J. Ultrasound Tumor Size Assessment, Histology and Serum Enzyme Analysis in a Rat Model of Colorectal Liver Cancer. Ultrasound Med. Biol. 2020, 46, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Khurshid, H.; Sankar, V.; Nemati, Z.; Phan, M.H.; Garayo, E.; García, J.A.; Srikanth, H. FeCo nanowires with enhanced heating powers and controllable dimensions for magnetic hyperthermia. J. Appl. Phys. 2015, 117, 17D113. [Google Scholar] [CrossRef]
- Garaio, E.; Sandre, O.; Collantes, J.-M.; Garcia, J.A.; Mornet, S.; Plazaola, F. Specific absorption rate dependence on temperature in magnetic field hyperthermia measured by dynamic hysteresis losses (ac magnetometry). Nanotechnology 2015, 26, 15704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaio, E.; Collantes, J.M.; Plazaola, F.; Garcia, J.A.; Castellanos-Rubio, I. A multifrequency eletromagnetic applicator with an integrated AC magnetometer for magnetic hyperthermia experiments. Meas. Sci. Technol. 2014, 25, 115702. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Insausti, M.; Garaio, E.; Gil de Muro, I.; Plazaola, F.; Rojo, T.; Lezama, L. Fe3O4 nanoparticles prepared by the seeded-growth route for hyperthermia: Electron magnetic resonance as a key tool to evaluate size distribution in magnetic nanoparticles. Nanoscale 2014, 12, 7542–7552. [Google Scholar] [CrossRef]
- Attaluri, A.; Ma, R.; Qiu, Y.; Li, W.; Zhu, L. Nanoparticle distribution and temperature elevations in prostatic tumours in mice during magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2011, 27, 491–502. [Google Scholar] [CrossRef]
- Dutz, S.; Kettering, M.; Hilger, I.; Müller, R.; Zeisberger, M. Magnetic multicore nanoparticles for hyperthermia—Influence of particle immobilization in tumour tissue on magnetic properties. Nanotechnology 2011, 22, 265102. [Google Scholar] [CrossRef]
- Alphandéry, E.; Faure, S.; Seksek, O.; Guyot, F.; Chebbi, I. Chains of Magnetosomes Extracted from AMB-1 Magnetotactic Bacteria for Application in Alternative Magnetic Field Cancer Therapy. ACS Nano 2011, 5, 6279–6296. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, D.; Liu, H.; Lai, R. Thermochemotherapy effect of nanosized As2O3/Fe3O4 complex on experimental mouse tumors and its influence on the expression of CD44v6, VEGF-C and MMP-9. BMC Biotechnol. 2009, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- van Landeghem, F.K.H.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-Mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEE Proc. Nanobiotechnol. 2004, 152, 33–39. [Google Scholar] [CrossRef]
- Shetake, N.; Pandey, B. Hyperthermia therapy of cancer: Need for deeper biological insights for improved therapeutic outcome. J. Radiat. Cancer Res. 2019, 10, 170–173. [Google Scholar] [CrossRef]
- Haveman, J.; Sminia, P.; Wondergem, J.; van der Zee, J.; Hulshof, M.C.C.M. Effects of hyperthermia on the central nervous system: What was learnt from animal studies? Int. J. Hyperth. 2005, 21, 473–487. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Viant, M.R. Revealing the Metabolome of Animal Tissues Using 1H Nuclear Magnetic Resonance Spectroscopy. In Metabolomics: Methods and Protocols; Weckwerth, W., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 358, pp. 229–246. [Google Scholar]
- Wu, H.; Southam, A.D.; Hines, A.; Viant, M.R. High-Throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef]
- Tuffnail, W.; Mills, G.A.; Cary, P.; Greenwood, R. An environmental 1H NMR metabolomic study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia and starvation. Metabolomics 2009, 5, 33–43. [Google Scholar] [CrossRef]
- Vinaixa, M.; Rodríguez, M.A.; Rull, A.; Beltrán, R.; Bladé, C.; Brezmes, J.; Cañellas, N.; Joven, J.; Correig, X. Metabolomic assessment of the effect of dietary cholesterol in the progressive development of fatty liver disease. J. Proteome Res. 2010, 9, 2527–2538. [Google Scholar] [CrossRef]
- Miao, Z.; Jin, M.; Liu, X.; Guo, W.; Jin, X.; Liu, H.; Wang, Y. The application of HPLC and microprobe NMR spectroscopy in the identification of metabolites in complex biological matrices. Anal. Bioanal. Chem. 2015, 407, 3405–3416. [Google Scholar] [CrossRef] [Green Version]
- Bollard, M.E.; Contel, N.R.; Ebbels, T.M.D.; Smith, L.; Beckonert, O.; Cantor, G.H.; Lehman-McKeeman, L.; Holmes, E.C.; Lindon, J.C.; Nicholson, J.K.; et al. NMR-Based metabolic profiling identifies biomarkers of liver regeneration following partial hepatectomy in the rat. J. Proteome Res. 2010, 9, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Amathieu, R.; Nahon, P.; Triba, M.; Bouchemal, N.; Trinchet, J.-C.; Beaugrand, M.; Dhonneur, G.; Le Moyec, L. Metabolomic approach by 1H NMR spectroscopy of serum for the assessment of chronic liver failure in patients with cirrhosis. J. Proteome Res. 2011, 10, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sengupta, A.; Sharma, S.; Sonawat, H.M. Metabolic Fingerprints of Serum, Brain, and Liver Are Distinct for Mice with Cerebral and Noncerebral Malaria: A 1H NMR Spectroscopy-Based Metabonomic Study. J. Proteome Res. 2012, 11, 4992–5004. [Google Scholar] [CrossRef] [PubMed]
- The Human Metabolome Database. Available online: http://www.hmdb.ca (accessed on 4 May 2016).
- Madison-Qingdao Metabolomics Consortium Database. Available online: http://mmcd.nmrfam.wisc.edu (accessed on 4 May 2016).
- Biological Magnetic Resonance Data Bank. Available online: http://www.bmrb.wisc.edu (accessed on 4 May 2016).
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.-Y.D.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef] [Green Version]
- Toriola, A.T.; Cheng, T.-Y.D.; Neuhouser, M.L.; Wener, M.H.; Zheng, Y.; Brown, E.; Miller, J.W.; Song, X.; Beresford, S.A.A.; Gunter, M.J.; et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int. J. Cancer 2013, 132, 2648–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmalek, M.F.; Sanderson, S.O.; Angulo, P.; Soldevila-Pico, C.; Liu, C.; Peter, J.; Keach, J.; Cave, M.; Chen, T.; McClain, C.J.; et al. Betaine for nonalcoholic fatty liver disease: Results of a randomized placebo-controlled trial. Hepatology 2009, 50, 1818–1826. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Morales, A.; Colell, A.; Ballesta, A.; Rodés, J.; Kaplowitz, N.; Fernández-Checa, J.C. Feeding S-adenosyl-l-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology 1995, 21, 207–214. [Google Scholar] [CrossRef]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov 2011, 10, 671–684. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Dang, C.V. Cancer’s Molecular Sweet Tooth and the Warburg Effect. Cancer Res. 2006, 66, 8927–8930. [Google Scholar] [CrossRef] [Green Version]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhao, L.; Ma, Z.; Chen, H. Metabolic reprogramming in cancer cells: Glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World J. Surg. Oncol. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Outschoorn, U.E.; Pavlides, S.; Sotgia, F.; Lisanti, M.P. Mitochondrial Biogenesis Drives Tumor Cell Proliferation. Am. J. Pathol. 2011, 178, 1949–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israël, M.; Schwartz, L. The metabolic advantage of tumor cells. Mol. Cancer 2011, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinig, R.E.; Clarke, E.F.; Waterhouse, C. Lactic acidosis and liver disease. Arch. Intern. Med. 1979, 139, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Panackel, C.; Thomas, R.; Sebastian, B.; Mathai, S.K. Recent advances in management of acute liver failure. Indian J. Crit. Care Med. 2015, 19, 27–33. [Google Scholar] [CrossRef]
- Feldman, A.G.; Sokol, R.J.; Hardison, R.M.; Alonso, E.M.; Squires, R.H.; Narkewicz, M.R. Lactate and Lactate: Pyruvate Ratio in the Diagnosis and Outcomes of Pediatric Acute Liver Failure. J. Pediatr. 2017, 182, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Debray, F.-G.; Mitchell, G.A.; Allard, P.; Robinson, B.H.; Hanley, J.A.; Lambert, M. Diagnostic Accuracy of Blood Lactate-to-Pyruvate Molar Ratio in the Differential Diagnosis of Congenital Lactic Acidosis. Clin. Chem. 2007, 53, 916–921. [Google Scholar] [CrossRef] [Green Version]
- Bolant Hernandez, B.; Calvo Bermudez, M.A.; Cejalvo Lapeña, D.; Gimeno Forner, O.; Gimeno Forner, L.; Lloris Carsi, J.M. Hematología y bioquímica clínica de la rata. Parte 1. Res. Surg. 1989, 3, 29–36. [Google Scholar]
- Bolant Hernandez, B.; Calvo Bermudez, M.A.; Cejalvo Lapeña, D.; Gimeno Forner, O.; Gimeno Forner, L.; Lloris Carsi, J.M. Hematología y bioquímica clínica de la rata. Parte 2. Res. Surg. 1990, 4, 12–20. [Google Scholar]
- Epstein, E.; Kiechle, F.L.; Artiss, J.D.; Zak, B. The clinical use of alkaline phosphatase enzymes. Clin. Lab. Med. 1986, 6, 491–505. [Google Scholar] [CrossRef]
- Cearra, I. Caracterización y Tratamiento Experimental del Síndrome de Isquemia-Reperfusión en Extremidades Inferiores en la Rata; Universidad del País Vasco UPV/EHU: Leioa-Erandio, Spain, 2016. [Google Scholar]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiele, F.; Vincent-Viry, M.; Fournier, B.; Starck, M.; Siest, G. Biological Effects of Eleven Combined Oral Contraceptives on Serum Triglycerides, γ-Glutamyltransferase, Alkaline Phosphatase, Bilirubin and other Biochemical Variables. Clin. Chem. Lab. Med. 1998, 36, 871–878. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero de la Parte, B.; Irazola, M.; Pérez-Muñoz, J.; Rodrigo, I.; Iturrizaga Correcher, S.; Mar Medina, C.; Castro, K.; Etxebarria, N.; Plazaola, F.; García, J.Á.; et al. Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats. Nanomaterials 2021, 11, 1318. https://doi.org/10.3390/nano11051318
Herrero de la Parte B, Irazola M, Pérez-Muñoz J, Rodrigo I, Iturrizaga Correcher S, Mar Medina C, Castro K, Etxebarria N, Plazaola F, García JÁ, et al. Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats. Nanomaterials. 2021; 11(5):1318. https://doi.org/10.3390/nano11051318
Chicago/Turabian StyleHerrero de la Parte, Borja, Mireia Irazola, Jorge Pérez-Muñoz, Irati Rodrigo, Sira Iturrizaga Correcher, Carmen Mar Medina, Kepa Castro, Nestor Etxebarria, Fernando Plazaola, Jose Ángel García, and et al. 2021. "Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats" Nanomaterials 11, no. 5: 1318. https://doi.org/10.3390/nano11051318
APA StyleHerrero de la Parte, B., Irazola, M., Pérez-Muñoz, J., Rodrigo, I., Iturrizaga Correcher, S., Mar Medina, C., Castro, K., Etxebarria, N., Plazaola, F., García, J. Á., García-Alonso, I., & Echevarría-Uraga, J. J. (2021). Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats. Nanomaterials, 11(5), 1318. https://doi.org/10.3390/nano11051318