Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of N,S-PCSs
2.2. MB Adsorption Experiments
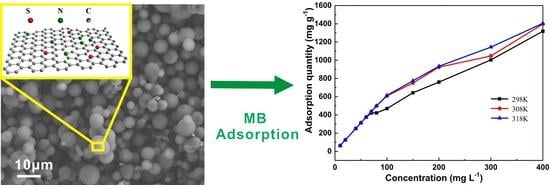
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, M.A.; Hilliou, L.; de Amorim, M.T.P. Fabrication of pristine-multiwalled carbon nanotubes/cellulose acetate composites for removal of methylene blue. Polym. Bull. 2020, 77, 623–653. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, D.T.C.; Le, H.T.N.; Duong, C.D.; Bach, L.G.; Nguyen, H.T.T.; Nguyen, T.D. Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere 2019, 227, 455–461. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, X.; Ding, H.; Li, Z.; Liang, R.; Sun, G. Villi-like poly(acrylic acid) based hydrogel adsorbent with fast highly efficient methylene blue removing ability. J. Colloid Interf. Sci. 2021, 594, 54–63. [Google Scholar] [CrossRef]
- Xiao, M.; Yue, H.D.; Feng, X.J.; Wang, Y.T.; He, M.T.; Chen, Q.; Zhang, Z.H. A double-layered neutral cadmium-organic framework for selective adsorption of cationic organic dyes through electrostatic affinity. J. Solid State Chem. 2020, 288, 121376. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, M.; Sun, S.; Zhao, H.; Meng, L.; Mu, T.; Song, J.; Jiang, N. Synergistic effect of MIL-88A/g-C3N4 and MoS2 to construct a self-cleaning multifunctional electrospun membrane. Chem. Eng. J. 2021, 3, 129621. [Google Scholar] [CrossRef]
- Udrea, M.L.; Paduretu, C.C.; Suica-Bunghez, I.R.; Doncea, S.M.; Ion, R.M. Methylene blue removal from residual water using fir wood sawdust as adsorbent. J. Sci. Arts 2021, 1, 261–272. [Google Scholar] [CrossRef]
- Min, H.S. Removal of Dyes from Wastewater by Adsorption onto Activated Carbon: Mini Review. J. Geosci. Environ. Prot. 2020, 8, 120–131. [Google Scholar]
- Zhou, X.; Chen, F.; Yang, J.; Ma, L.; Bai, T.; Long, B.; Liao, Q.; Liu, C. Dual protection of sulfur by interconnected porous carbon nanorods graphene sheets for lithium–sulfur batteries. J. Electroanal. Chem. 2015, 747, 59–67. [Google Scholar] [CrossRef]
- Jia, R.; Chen, J.; Zhao, J.; Zheng, J.; Song, C.; Li, L.; Zhu, Z. Synthesis of highly nitrogen-doped hollow carbon nanoparticles their excellent electrocatalytic properties in dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 10829–10834. [Google Scholar] [CrossRef]
- Kang, Y.; Guo, Z.; Zhang, J.; Xie, H.; Liu, H.; Zhang, C. Enhancement of Ni(II) removal by urea-modified activated carbon derived from Pennisetum alopecuroides with phosphoric acid activation. J. Taiwan Inst. Chem. E 2016, 60, 335–341. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Lv, L.; Chen, S.; Sun, W.; Wang, Y. Two-dimensional imine-based covalent-organic-framework derived nitrogen-doped porous carbon nanosheets for high-performance lithium-sulfur batteries. New J. Chem. 2021, 45, 8683. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Ma, L.; Ren, J.; Ma, P.; Li, B.; Wu, N.; Song, Z.; Huang, L. Nitrogen and sulfur codoped micro-mesoporous carbon sheets derived from natural biomass for synergistic removal of chromium (VI): Adsorption behavior and computing mechanism. Sci. Total Environ. 2020, 730, 138930. [Google Scholar] [CrossRef]
- Zhuang, Q.Q.; Cao, J.P.; Wu, Y.; Zhao, M.; Zhao, X.Y.; Zhao, Y.P.; Bai, H.C. Heteroatom nitrogen and oxygen co-doped three-dimensional honeycomb porous carbons for methylene blue efficient removal. Appl. Surf. Sci. 2021, 546, 149139. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Ma, G.; Kong, D.; Xiong, W.; Wang, J.; Zhu, Y.; Xia, Y. Heteroatom-doped porous carbons with enhanced carbon dioxide uptake and excellent methylene blue adsorption capacities. Microporous Mesoporous Mater. 2018, 257, 1–8. [Google Scholar] [CrossRef]
- Hu, X.; Min, X.; Wang, H.; Li, X.; He, Y.; Yang, W. Enhanced capacitive deionization boosted by Co and N co-doping in carbon materials. Sep. Purif. Technol. 2021, 266, 118590. [Google Scholar]
- Zhang, H.; Zhou, W.; Huang, D.; Ou, L.; Lan, Z.; Liang, X.; Huang, H.; Huang, D.; Guo, J. Functionalized hierarchical porous carbon with sulfur/nitrogen/oxygen tri-doped as high quality sulfur hosts for lithium-sulfur batteries. J. Alloys Compd. 2020, 858, 157647. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yang, W.; An, X.; Sun, A.; Fan, X.; Pan, Q. In situ modification of ZIF-67 with multi-sulfonated dyes for great enhanced methylene blue adsorption via synergistic effect. Micropor. Mesopor. Mat. 2020, 303, 110304. [Google Scholar] [CrossRef]
- Jeng, H.B.; Teck, H.L.; Jin, H.L.; Lai, J.C. Cellulose nanofibril-based aerogel derived from sago pith waste its application on methylene blue removal. Int. J. Biol. Macromol. 2020, 160, 836–845. [Google Scholar]
- Zhou, X.; Wang, P.; Zhang, Y.; Wang, L.; Zhang, L.; Zhang, L.; Xu, L.; Liu, L. Biomass based nitrogen-doped structure-tunable versatile porous carbon materials. J. Mater. Chem. A 2017, 5, 12958–12968. [Google Scholar] [CrossRef]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.H.; Kang, F. N and S co-doped porous carbon spheres prepared using L-cysteine as a dual functional agent for high-performance lithium-sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, S.; Huang, C.; Wang, X.; Cai, S.; Xiang, K.; Zhang, Y.; Xue, J. Honeycomb-like Nitrogen and Sulfur Dual-doped Hierarchical Porous Biomass Carbon for High-energy-density Lithium-sulfur Batteries. ChemSusChem 2017, 10, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Hu, Z.; Tong, C.; Zhang, Z.; Hu, S. Carbon dots codoped with nitrogen and sulfur are viable fluorescent probes for chromium(VI). Microchim. Acta 2017, 184, 1547–1553. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.H.; Wu, G.H.; Zhang, J.R.; Zhu, J.J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Gong, J.; Lin, H.; Antonietti, M.; Yuan, J. Nitrogen-doped porous carbon nanosheets derived from poly(ionic liquid): Hierarchical pore structures for efficient CO2 capture dye removal. J. Mater. Chem. A 2016, 4, 7313. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, L.; Gao, Y.; Li, A. Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb(II), Cu(II) and Cr(VI) removals. Waste Manag. 2018, 79, 625–637. [Google Scholar] [CrossRef]
- Sriramoju, S.K.; Dash, P.S.; Majumdar, S. Meso-porous activated carbon from lignite waste and its application in methylene Blue adsorption and coke plant effluent treatment. J. Environ. Chem. Eng. 2021, 9, 104784. [Google Scholar] [CrossRef]
- Thta, H.T.; Le, H.H.; Pham, T.H.; Nguyen, D.T.; La, D.D.; Chang, S.W.; Lee, S.M.; Chung, W.J.; Nguyen, D.D. Comparative study on methylene blue adsorption behavior of coffee husk-derived activated carbon materials prepared using hydrothermal soaking methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar]
- Jiang, L.; Wen, Y.; Zhu, Z.; Liu, X.; Shao, W. A Double cross-linked strategy to construct graphene aerogels with highly efficient methylene blue adsorption performance. Chemosphere 2021, 265, 129169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Li, M.; Zheng, H.; Zhang, X.; Li, H.; DU, Q. Methylene blue adsorption by activated carbon, nickel alginate/activated carbon aerogel, and nickel alginate/graphene oxide aerogel: A comparison study. J. Mater. Res. Technol. 2020, 9, 12443–12460. [Google Scholar] [CrossRef]
- Thi, H.T.; Hoang, A.L.; Huu, T.P.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hua, Z.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; WU, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Nguyen, V.T.; Dung, N.Q.; Chu, M.N.; Kiet, D.V.; Ngan, T.H.K.; Tan, L.V. Study on methylene blue adsorption of activated carbon made from Moringa oleifera leaf. Mater. Today Proc. 2021, 38, 3405–3413. [Google Scholar] [CrossRef]
- Asuha, S.; Fei, F.; Wurendaodi, W.; Zhuang, X. Activation of kaolinite by a low-temperature chemical method and its effect on methylene blue adsorption. Powder Technol. 2020, 361, 624–632. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.; Li, Y. Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties. Materials 2020, 13, 4407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, L.; Xia, H.; Peng, J.; Shu, J.; Li, C.; Jiang, X.; Zhang, Q. Adsorption behavior of methylene blue onto waste-derived adsorbent and exhaust gases recycling. RSC Adv. 2017, 7, 27331–27341. [Google Scholar] [CrossRef] [Green Version]
- Alver, E.; Metin, A.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Ren, J.; Luo, X.; Liu, B.; Zhou, X. Sandwich-Type Nitrogen and Sulfur Codoped Graphene-Backboned Porous Carbon Coated Separator for High Performance Lithium-Sulfur Batteries. Nanomaterials 2018, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, Y.; Zhang, X.; Zheng, H.; Zhang, A.; Chen, T.; Liu, W.; Yu, Y.; Liu, J.; Du, Q.; et al. One-step generation of S and N co-doped reduced graphene oxide for high-efficiency adsorption towards methylene blue. RSC Adv. 2020, 10, 37757–37765. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Ren, J.; Luo, X.; Bing, L.; Song, Z.; Zhou, X. Wheat Straw-Derived N-, O-, and S-Tri-doped Porous Carbon with Ultrahigh Specific Surface Area for Lithium-Sulfur Batteries. Materials 2018, 11, 989. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.Q.; Wang, Z.W.; Wu, P.C.; Wang, Y.; Fu, J. Mass fabrication of oxygen and nitrogen co-doped 3D hierarchical porous carbon nanosheets by an explosionassisted strategy for supercapacitor and dye adsorption application. Appl. Surf. Sci. 2020, 529, 147079. [Google Scholar] [CrossRef]








| Samples | Type | Elemental Contents | |
|---|---|---|---|
| N | S | ||
| N,S-PCSs-1 | Weight percentage | 0.39 | 1.05 |
| (Atomic percentage) | −0.34 | −0.4 | |
| N,S-PCSs-2 | Weight percentage | 0.98 | 6.7 |
| (Atomic percentage) | −0.92 | −2.75 | |
| N,S-PCSs-3 | Weight percentage | 1.71 | 8.24 |
| (Atomic percentage) | −1.57 | −3.3 | |
| (Model) Equation | Fitted Parameter | Fitted Equation | ||
|---|---|---|---|---|
| Adsorption kinetics | (Pseudo-second-order) x = t, y = t/qt | k2 | 0.00067 | y = 0.0023x + 0.0079 |
| qe (mg g−1) | 434.78 | |||
| qexp (mg g−1) | 433.01 | |||
| R2 | 0.9999 | |||
| (Internal diffusion) x = t1/2, y = qt | (Stage 1) kp | 15.76 | y = 15.756x + 299.18 | |
| (Stage 1) R2 | 0.9469 | |||
| (Stage 2) kp | 6.25 | y = 6.2491x + 343.16 | ||
| (Stage 2) R2 | 0.9446 | |||
| (Stage 3) kp | −0.011 | y = −0.0106x + 433.24 | ||
| (Stage 3) R2 | 0.9786 | |||
| Adsorption isotherms | (Langmuir model) x = Ce y = Ce/qe | qm (mg g−1) | 909.10 | y = 0.0011x + 0.0073 |
| b | 0.15 | |||
| R2 | 0.9410 | |||
| (Freundlich model) x = lg Ce, y = lg qe | k | 411.81 | y = 0.1237x + 2.6147 | |
| 1/n | 0.12 | |||
| R2 | 0.7720 | |||
| ΔHθ (kJ mol−1) | ΔSθ (kJ mol−1 k−1) | ΔGθ (kJ mol−1) | ||
|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||
| 5.57 | 19.76 | −0.27 | −0.61 | −0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Chen, F.; Pan, K.; Zhao, Y.; Ma, L.; Wei, S. Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres. Nanomaterials 2021, 11, 1819. https://doi.org/10.3390/nano11071819
Ren Y, Chen F, Pan K, Zhao Y, Ma L, Wei S. Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres. Nanomaterials. 2021; 11(7):1819. https://doi.org/10.3390/nano11071819
Chicago/Turabian StyleRen, Yongpeng, Feng Chen, Kunming Pan, Yang Zhao, Lulu Ma, and Shizhong Wei. 2021. "Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres" Nanomaterials 11, no. 7: 1819. https://doi.org/10.3390/nano11071819
APA StyleRen, Y., Chen, F., Pan, K., Zhao, Y., Ma, L., & Wei, S. (2021). Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres. Nanomaterials, 11(7), 1819. https://doi.org/10.3390/nano11071819