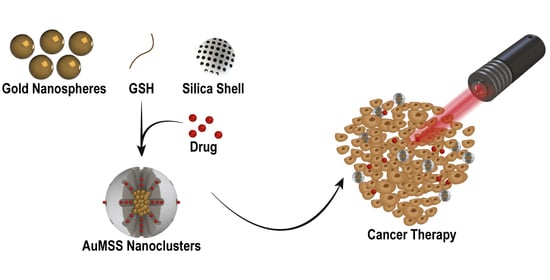
Optimization of the GSH-Mediated Formation of Mesoporous Silica-Coated Gold Nanoclusters for NIR Light-Triggered Photothermal Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AuMSS Nanoclusters
2.3. Characterization of Nanocarriers’ Physicochemical Properties
2.4. In Vitro Photothermal Measurements
2.5. Cytocompatibility Assay
2.6. Characterization of the Nanoclusters Phototherapeutic Effect
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the GSH Effect on the Agglomeration of Gold Nanoparticles
3.2. Optimization of the Mesoporous Silica Shell
3.3. In Vitro Evaluation of the AuMSS Nanoclusters Photothermal Capacity
3.4. AuMSS Nanoclusters Cytocompatibility
3.5. Photothermal Effect Mediated by AuMSS Nanoclusters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29, 1604894. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Jacinto, T.A.; Moreira, A.F.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Functionalization of AuMSS nanorods towards more effective cancer therapies. Nano Res. 2019, 12, 719–732. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, Z.; Telang, S.; Ramchand, C. Application of magnetic techniques in the field of drug discovery and biomedicine. BioMagnetic Res. Technol. 2003, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and t2* magnetic resonance imaging. Investig. Radiol. 2011, 46, 132. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Strategies to improve the photothermal capacity of gold-based nanomedicines. Acta Biomater. 2020, 116, 105–137. [Google Scholar] [CrossRef]
- Leitão, M.M.; de Melo-Diogo, D.; Alves, C.G.; Lima-Sousa, R.; Correia, I.J. Prototypic Heptamethine Cyanine Incorporating Nanomaterials for Cancer Phototheragnostic. Adv. Healthc. Mater. 2020, 9, 1901665. [Google Scholar] [CrossRef]
- Chen, Y.; Khan, A.R.; Yu, D.; Zhai, Y.; Ji, J.; Shi, Y.; Zhai, G. Pluronic F127-functionalized molybdenum oxide nanosheets with pH-dependent degradability for chemo-photothermal cancer therapy. J. Colloid Interface Sci. 2019, 553, 567–580. [Google Scholar] [CrossRef]
- Dong, H.; Cao, Y. Carbon Nanomaterials for Optical Bioimaging and Phototherapy. Carbon Nanomater. Bioimaging Bioanal. Ther. 2019, 43–62. [Google Scholar]
- Estelrich, J.; Busquets, M.A. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Correia, I.J. Gold-core silica shell nanoparticles application in imaging and therapy: A review. Microporous Mesoporous Mater. 2018, 270, 168–179. [Google Scholar] [CrossRef]
- Yin, S.; Asakura, Y. Recent research progress on mixed valence state tungsten based materials. Tungsten 2019, 1, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.A.; Rodrigues, C.F.; Moreira, A.F.; Jacinto, T.A.; Ferreira, P.; Correia, I.J. Development of gold-core silica shell nanospheres coated with poly-2-ethyl-oxazoline and β-cyclodextrin aimed for cancer therapy. Mater. Sci. Eng. C 2019, 98, 960–968. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Wang, L.; Liu, G.; Cheng, Y.; Lin, M.; Sui, K.; Zhang, H. Alginate mediated functional aggregation of gold nanoclusters for systemic photothermal therapy and efficient renal clearance. Carbohydr. Polym. 2020, 241, 116344. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C.; Park, S.; Lim, K.; Kim, S.S.; Kim, J.O.; Lee, E.S.; Oh, K.T.; Choi, H.; Youn, Y.S. Facile fabrication of highly photothermal-effective albumin-assisted gold nanoclusters for treating breast cancer. Int. J. Pharm. 2018, 553, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Lim, S.C.; Lim, K.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation. J. Control. Release 2019, 304, 7–18. [Google Scholar] [CrossRef]
- Iodice, C.; Cervadoro, A.; Palange, A.; Key, J.; Aryal, S.; Ramirez, M.R.; Mattu, C.; Ciardelli, G.; O’Neill, B.E.; Decuzzi, P. Enhancing photothermal cancer therapy by clustering gold nanoparticles into spherical polymeric nanoconstructs. Opt. Lasers Eng. 2016, 76, 74–81. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Deng, Y.; Zeng, M.; Tang, Y.; Zhu, W.-H.; Cheng, Y. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials 2017, 139, 30–38. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Universal scaling of plasmon coupling in metal nanostructures: Extension from particle pairs to nanoshells. Nano Lett. 2007, 7, 2854–2858. [Google Scholar] [CrossRef]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Asim Farooq, M.; Aquib, M.; Maviah Mily, B.J.; Murtaza, G. Mesoporous silica nanomaterials: Versatile nanocarriers for cancer theranostics and drug and gene delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Hanafi-Bojd, M.Y.; Jaafari, M.R.; Ramezanian, N.; Xue, M.; Amin, M.; Shahtahmassebi, N.; Malaekeh-Nikouei, B. Surface functionalized mesoporous silica nanoparticles as an effective carrier for epirubicin delivery to cancer cells. Eur. J. Pharm. Biopharm. 2015, 89, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Montoto, A.H.; Montes, R.; Samadi, A.; Gorbe, M.; Terrés, J.M.; Cao-Milan, R.; Aznar, E.; Ibanez, J.; Masot, R.; Marcos, M.D. Gold nanostars coated with mesoporous silica are effective and nontoxic photothermal agents capable of gate keeping and laser-induced drug release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef]
- Guimarães, R.S.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of stimuli-responsive mesoporous organosilica nanocarriers for drug delivery. Pharmacol. Res. 2020, 155, 104742. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Ferreira, P.; Correia, I.J. Development of poly-2-ethyl-2-oxazoline coated gold-core silica shell nanorods for cancer chemo-photothermal therapy. Nanomedicine 2018, 13, 2611–2627. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montoto, A.; Gorbe, M.; Llopis-Lorente, A.; Terrés, J.M.; Montes, R.; Cao-Milán, R.; De Greñu, B.D.; Alfonso, M.; Orzaez, M.; Marcos, M.D. A NIR light-triggered drug delivery system using core–shell gold nanostars–mesoporous silica nanoparticles based on multiphoton absorption photo-dissociation of 2-nitrobenzyl PEG. Chem. Commun. 2019, 55, 9039–9042. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, Y.; Shi, J.; Ma, T.; Zhang, Z.; Ma, H.; Cao, S. Hydroxyapatite/mesoporous silica coated gold nanorods with improved degradability as a multi-responsive drug delivery platform. Mater. Sci. Eng. C 2018, 83, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Kim, B.; Auguste, D.T. Incorporating gold nanoclusters and target-directed liposomes as a synergistic amplified colorimetric sensor for HER2-positive breast cancer cell detection. Theranostics 2017, 7, 899. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold nanoparticles aggregation: Drastic effect of cooperative functionalities in a single molecular conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Stobiecka, M.; Deeb, J.; Hepel, M. Ligand exchange effects in gold nanoparticle assembly induced by oxidative stress biomarkers: Homocysteine and cysteine. Biophys. Chem. 2010, 146, 98–107. [Google Scholar] [CrossRef]
- Stobiecka, M.; Coopersmith, K.; Hepel, M. Resonance elastic light scattering (RELS) spectroscopy of fast non-Langmuirian ligand-exchange in glutathione-induced gold nanoparticle assembly. J. Colloid Interface Sci. 2010, 350, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M.; Stobiecka, M. Detection of oxidative stress biomarkers using functional gold nanoparticles. In Fine Particles in Medicine and Pharmacy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 241–281. [Google Scholar]
- Zhang, B.; Wei, L.; Chu, Z. Development of indocyanine green loaded Au@ Silica core shell nanoparticles for plasmonic enhanced light triggered therapy. J. Photochem. Photobiol. A Chem. 2019, 375, 244–251. [Google Scholar] [CrossRef]
- Fernández-López, C.; Mateo-Mateo, C.; Alvarez-Puebla, R.A.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Highly controlled silica coating of PEG-capped metal nanoparticles and preparation of SERS-encoded particles. Langmuir 2009, 25, 13894–13899. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.R.; Moreira, A.F.; Correia, I.J. The effect of the shape of gold core–mesoporous silica shell nanoparticles on the cellular behavior and tumor spheroid penetration. J. Mater. Chem. B 2016, 4, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Reis, C.A.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Optimization of gold core-mesoporous silica shell functionalization with TPGS and PEI for cancer therapy. Microporous Mesoporous Mater. 2019, 285, 1–12. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y.; Lee, H.; Lee, M.; An, J.Y.; Shin, S.; Kwon, S.; Lee, D.S.; Park, J.H. Gold-Nanoclustered Hyaluronan Nano-Assemblies for Photothermally Maneuvered Photodynamic Tumor Ablation. ACS Nano 2016, 10, 10858–10868. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regi, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Li, M.Q.; Lao, Y.H.; Mintz, R.L.; Chen, Z.G.; Shao, D.; Hu, H.Z.; Wang, H.X.; Tao, Y.; Leong, K.W. A multifunctional mesoporous silica-gold nanocluster hybrid platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay. Nanoscale 2019, 11, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Rodrigues, C.F.; Moreira, A.F.; Miguel, S.P.; Costa, E.C.; Ferreira, P.; Correia, I.J. Hyaluronic acid and vitamin e polyethylene glycol succinate functionalized gold-core silica shell nanorods for cancer targeted photothermal therapy. Colloids Surf. B Biointerfaces 2020, 188, 110778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Mero, A.; Pasut, G.; Dalla Via, L.; Fijten, M.W.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. Synthesis and characterization of poly (2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef]
- He, Q.; Gao, Y.; Zhang, L.; Zhang, Z.; Gao, F.; Ji, X.; Li, Y.; Shi, J. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials 2011, 32, 7711–7720. [Google Scholar] [CrossRef]
- Jia, Y.P.; Shi, K.; Liao, J.F.; Peng, J.R.; Hao, Y.; Qu, Y.; Chen, L.J.; Liu, L.; Yuan, X.; Qian, Z.Y. Effects of Cetyltrimethylammonium Bromide on the Toxicity of Gold Nanorods both In Vitro and In Vivo: Molecular Origin of Cytotoxicity and Inflammation. Small Methods 2020, 4, 1900799. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, Y.; Ji, W.; Ye, W.; Jiang, Y.; Song, J. Inhibitation of cellular toxicity of gold nanoparticles by surface encapsulation of silica shell for hepatocarcinoma cell application. ACS Appl. Mater. Interfaces 2014, 6, 19327–19335. [Google Scholar] [CrossRef]
- Chan, M.-H.; Lin, H.-M. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual-mode imaging, theranostics, and targeted tracking. Biomaterials 2015, 46, 149–158. [Google Scholar] [CrossRef] [PubMed]
- De Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to improve cancer photothermal therapy mediated by nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, N.; Rodrigues, C.F.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Optimization of the GSH-Mediated Formation of Mesoporous Silica-Coated Gold Nanoclusters for NIR Light-Triggered Photothermal Applications. Nanomaterials 2021, 11, 1946. https://doi.org/10.3390/nano11081946
Fernandes N, Rodrigues CF, de Melo-Diogo D, Correia IJ, Moreira AF. Optimization of the GSH-Mediated Formation of Mesoporous Silica-Coated Gold Nanoclusters for NIR Light-Triggered Photothermal Applications. Nanomaterials. 2021; 11(8):1946. https://doi.org/10.3390/nano11081946
Chicago/Turabian StyleFernandes, Natanael, Carolina F. Rodrigues, Duarte de Melo-Diogo, Ilídio J. Correia, and André F. Moreira. 2021. "Optimization of the GSH-Mediated Formation of Mesoporous Silica-Coated Gold Nanoclusters for NIR Light-Triggered Photothermal Applications" Nanomaterials 11, no. 8: 1946. https://doi.org/10.3390/nano11081946
APA StyleFernandes, N., Rodrigues, C. F., de Melo-Diogo, D., Correia, I. J., & Moreira, A. F. (2021). Optimization of the GSH-Mediated Formation of Mesoporous Silica-Coated Gold Nanoclusters for NIR Light-Triggered Photothermal Applications. Nanomaterials, 11(8), 1946. https://doi.org/10.3390/nano11081946