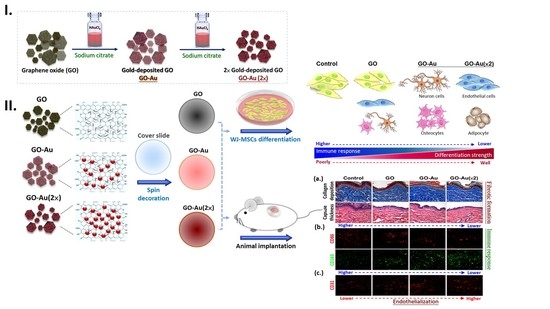
Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GO-Au Nanocomposites
2.2. Characterization Methods
2.3. Free Radical Scavenging Assay
2.4. Cell Culture
2.5. Characterization of Mesenchymal Stem Cells
2.6. Cell Viability Assay
2.7. Monocyte and Platelet Activation Assay
2.8. Determination of Intracellular Reactive Oxygen Species Generation
2.9. Enzyme-Linked Immunosorbent Assay (ELISA Assay)
2.10. Metalloproteinases Zymography Assay
2.11. Immunofluorescence Staining Assay
2.12. Alizarin Red S (ARS) Staining
2.13. Oil Red O (ORO) Staining Assay
2.14. In Vivo Biocompatibility Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Physicochemical Properties of Au-Decorated GO Nanocomposites
3.2. Cytocompatibility of Go-Au in MSCs
3.3. Anti-Inflammatory Effect of GO-Au
3.4. Enhanced Differentiation Ability of MSCs by GO-Au
3.5. In Vivo Biocompatibility and Anti-Inflammatory Effect of GO-Au
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Persidis, A. Tissue engineering. Nat. Biotechnol. 1999, 17, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chua, C.K.; Yu, T.; Li, H.; Tan, L.P. Advanced nanobiomaterial strategies for the development of organized tissue engineering constructs. Nanomedicine 2013, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar]
- Chandra, P.K.; Soker, S.; Atala, A. Chapter 1—Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–35. [Google Scholar]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef]
- Han, S.; Sun, J.; He, S.; Tang, M.; Chai, R. The application of graphene-based biomaterials in biomedicine. Am. J. Transl. Res. 2019, 11, 3246–3260. [Google Scholar]
- Anzalone, R.; Iacono, M.L.; Corrao, S.; Magno, F.; Loria, T.; Cappello, F.; Zummo, G.; Farina, F.; La Rocca, G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010, 19, 423–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-W.; Hsieh, S.-C.; Yang, Y.-C.; Hsu, S.-H.; Kung, M.-L.; Lin, P.-Y.; Hsieh, H.-H.; Lin, C.-H.; Tang, C.-M.; Hung, H.-S. Functional engineered mesenchymal stem cells with fibronectin-gold composite coated catheters for vascular tissue regeneration. Nanomedicine 2018, 14, 699–711. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Chen, H.-J.; Hsu, S.-H.; Yang, Y.-C.; Tang, C.-M.; Chu, M.-Y.; Lin, P.-Y.; Fu, R.-H.; Kung, M.-L.; Chen, Y.-W.; et al. Prominent Vascularization Capacity of Mesenchymal Stem Cells in Collagen-Gold Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28982–29000. [Google Scholar] [CrossRef]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic Nanocomposite Hydrogel for Potential Cartilage Tissue Engineering: Synthesis, Characterization, and Cytocompatibility with Bone Marrow Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef]
- Singla, R.; Abidi, S.M.S.; Dar, A.; Acharya, A. Nanomaterials as potential and versatile platform for next generation tissue engineering applications. J. Biomed Mater. Res. B Appl. Biomater. 2019, 107, 2433–2449. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Dasari Shareena, T.P.; McShan, D.; DasMahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Menaa, F.; AbdelGhani, A.; Menaa, B. Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: Impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regen Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Kim, F.; Cote, L.J.; Huang, J. Graphene Oxide: Surface Activity and Two-Dimensional Assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef]
- Sydlik, S.; Jhunjhunwala, S.; Webber, M.; Anderson, D.G.; Langer, R.S. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-H.; Lee, T.; El-Said, W.A.; Choi, J.-W. Graphene-Based Materials for Stem Cell Applications. Materials 2015, 8, 8674–8690. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef]
- Nayak, T.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene nanogrids for selective and fast osteogenic differentiation of human mesenchymal stem cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.-H.; Yu, A.; Yeh, C.-A.; Sun, W.-S.; Lin, S.-Z.; Fu, R.-H.; Hsieh, H.-H.; Wu, P.-Y.; Hung, H.-S.; Yu, Y.-H. Biocompatible Nanogold Carrier Coated with Hyaluronic Acid for Efficient Delivery of Plasmid or siRNA to Mesenchymal Stem Cells. ACS Appl. Bio Mater. 2019, 2, 1017–1030. [Google Scholar] [CrossRef]
- Hung, H.-S.; Yang, Y.-C.; Lin, Y.-C.; Lin, S.-Z.; Kao, W.-C.; Hsieh, H.-H.; Chu, M.-Y.; Fu, R.-H.; Hsu, S.-H. Regulation of human endothelial progenitor cell maturation by polyurethane nanocomposites. Biomaterials 2014, 35, 6810–6821. [Google Scholar] [CrossRef]
- SKumar, S.; Chatterjee, K. Comprehensive Review on the Use of Graphene-Based Substrates for Regenerative Medicine and Biomedical Devices. ACS Appl. Mater. Interfaces 2016, 8, 26431–26457. [Google Scholar]
- Chuang, M.-K.; Chen, F.-C.; Hsu, C.-S. Gold Nanoparticle-Graphene Oxide Nanocomposites That Enhance the Device Performance of Polymer Solar Cells. J. Nanomater. 2014, 2014, 736879. [Google Scholar] [CrossRef]
- Pendolino, F.; Capurso, G.; Maddalena, A.; Russo, S.L. The structural change of graphene oxide in a methanol dispersion. RSC Adv. 2014, 4, 32914–32917. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, C.H.; Ho, T.T.; Chen, H.C.; Chu, M.Y.; Sun, W.S.; Kao, W.C.; Hung, H.S.; Hsu, S.-H. Enhanced Migration of Wharton’s Jelly Mesenchymal Stem Cells Grown on Polyurethane Nanocomposites. J. Med. Biol. Eng. 2013, 33, 139–148. [Google Scholar] [CrossRef]
- Hung, H.-S.; Chang, C.-H.; Chang, C.-J.; Tang, C.-M.; Kao, W.-C.; Lin, S.-Z.; Hsieh, H.-H.; Chu, M.-Y.; Sun, W.-S.; Hsu, S.-H. In Vitro Study of a Novel Nanogold-Collagen Composite to Enhance the Mesenchymal Stem Cell Behavior for Vascular Regeneration. PLoS ONE 2014, 9, e104019. [Google Scholar]
- Hsu, S.-H.; Tang, C.-M.; Tseng, H.-J. Biocompatibility of poly(ether)urethane-gold nanocomposites. J. Biomed. Mater. Res. A 2006, 79, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, M.A. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.-C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Yu, H.; Xu, P.; Lee, D.-W.; Li, X. Porous-layered stack of functionalized AuNP–rGO (gold nanoparticles–reduced graphene oxide) nanosheets as a sensing material for the micro-gravimetric detection of chemical vapor. J. Mater. Chem. A 2013, 1, 4444–4450. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L.; Chang, D.W.; Baek, J.-B. Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef]
- Polini, A.; Yang, F. 5—Physicochemical characterization of nanofiber composites. In Nanofiber Composites for Biomedical Applications; Ramalingam, M., Ramakrishna, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 97–155. [Google Scholar]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; Van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [Green Version]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Hung, H.-S.; Tang, C.-M.; Lin, C.-H.; Lin, S.-Z.; Chu, M.-Y.; Sun, W.-S.; Kao, W.-C.; Hsien-Hsu, H.; Huang, C.-Y.; Hsu, S.-H. Biocompatibility and favorable response of mesenchymal stem cells on fibronectin-gold nanocomposites. PLoS ONE 2013, 8, e65738. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, B.; Han, J.; Oh, J.; Park, S.; Ryu, S.; Jung-Youn, S.; Shin, J.-Y.; Lee, B.S.; Hong, B.H.; et al. Graphene oxide flakes as a cellular adhesive: Prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano 2015, 9, 4987–4999. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Cencioni, C.; Capogrossi, M.C.; Napolitano, M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc. Res. 2012, 94, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.J.; Faustino, R.S.; Chiriac, A.; Crespo-Diaz, R.; Behfar, A.; Terzic, A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells 2008, 26, 1464–1473. [Google Scholar] [CrossRef]
- Gupta, S.K.; Lysko, P.G.; Pillarisetti, K.; Ohlstein, E.; Stadel, J.M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 1998, 273, 4282–4287. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Schober, A.; Bot, I.; von Hundelshausen, P.; Liehn, E.A.; Möpps, B.; Mericskay, M.; Gierschik, P.; Biessen, E.A.L.; Weber, C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 2005, 96, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Broxmeyer, H.E. Chemokines in hematopoiesis. Curr. Opin. Hematol. 2008, 15, 49–58. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the Migration Ability of Mesenchymal Stromal Cells by Targeting the SDF-1/CXCR4 Axis. BioMed. Res. Int. 2013, 2013, 561098. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, S.; Li, Y.; Wang, X.; Xue, W.; Ge, G.; Luo, X. The Role of SDF-1-CXCR4/CXCR7 Axis in the Therapeutic Effects of Hypoxia-Preconditioned Mesenchymal Stem Cells for Renal Ischemia/Reperfusion Injury. PLoS ONE 2012, 7, e34608. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.-I.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Tachibana, K.; Hirota, S.; Iizasa, H.; Yoshida, H.; Kawabata, K.; Kataoka, Y.; Kitamura, Y.; Matsushima, K.; Yoshida, N.; Nishikawa, S.-I.; et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998, 393, 591–594. [Google Scholar] [CrossRef]
- YZou, Y.-R.; Kottmann, A.; Kuroda, M.; Taniuchi, I.; Littman, D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998, 393, 595–599. [Google Scholar]
- Kawaguchi, N.; Zhang, T.-T.; Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; An, J.; Han, X.; Zhang, X.; Wang, W.; Wang, S. Hypermethylation of microRNA-149 activates SDF-1/CXCR4 to promote osteogenic differentiation of mesenchymal stem cells. J. Cell Physiol. 2019, 234, 23485–23494. [Google Scholar] [CrossRef]
- Ho, S.Y.; Ling, T.Y.; Lin, H.Y.; Liou, J.T.; Liu, F.C.; Chen, I.C.; Lee, S.W.; Hsu, Y.; Lai, D.M.; Liou, H.H. SDF-1/CXCR4 Signaling Maintains Stemness Signature in Mouse Neural Stem/Progenitor Cells. Stem Cells Int. 2017, 2017, 2493752. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.; Ma, M.; Vegas, A.F.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 2, 2941–2953. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.; Ng, J.; Nakazawa, K.R.; Daulton, J.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [Green Version]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant Potential and Toxicity Study of the Cerium Oxide Nanoparticles Synthesized by Microwave-Mediated Synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.V.; Petronilho, F.; Pereira, H.R.S.B.; Vuolo, F.; Mina, F.; Possato, J.C.; Vitto, M.F.; de Souza, D.R.; da Silva, L.; Paula, M.M.D.S.; et al. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8036–8041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.S.; Kim, W.J.; Kim, J.J.; Kim, T.J.; Ye, S.K.; Song, M.D.; Kang, H.; Kim, D.W.; Moon, W.K.; Lee, K.H. Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-κB and IFN-β/STAT1 pathways in RAW264.7 cells. Nitric Oxide 2010, 23, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.C.; Venâncio, M.; Souza, P.S.; Victor, E.G.; Notoya, F.D.S.; Paganini, C.S.; Streck, E.L.; da Silva, L.; Pinho, R.; Paula, M.M. Iontophoresis with gold nanoparticles improves mitochondrial activity and oxidative stress markers of burn wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 380–385. [Google Scholar] [CrossRef]
- Uchiyama, M.K.; Deda, D.K.; Rodrigues, S.F.; Drewes, C.C.; Bolonheis, S.M.; Kiyohara, P.K.; Toledo, S.P.; Colli, W.; Araki, K.; Farsky, S.H. In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol. Sci. 2014, 142, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Seizer, P.; Gawaz, M.; May, A. Platelet-monocyte interactions—A dangerous liaison linking thrombosis, inflammation and atherosclerosis. Curr. Med. Chem. 2008, 15, 1976–1980. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. The pathogenesis of atherosclerosis (first of two parts). N. Engl. J. Med. 1976, 295, 369–377. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. The pathogenesis of atherosclerosis (second of two parts). N. Engl. J. Med. 1976, 295, 420–425. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-S.; Kung, M.-L.; Chen, F.-C.; Ke, Y.-C.; Shen, C.-C.; Yang, Y.-C.; Tang, C.-M.; Yeh, C.-A.; Hsieh, H.-H.; Hsu, S.-h. Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials 2021, 11, 2046. https://doi.org/10.3390/nano11082046
Hung H-S, Kung M-L, Chen F-C, Ke Y-C, Shen C-C, Yang Y-C, Tang C-M, Yeh C-A, Hsieh H-H, Hsu S-h. Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials. 2021; 11(8):2046. https://doi.org/10.3390/nano11082046
Chicago/Turabian StyleHung, Huey-Shan, Mei-Lang Kung, Fang-Chung Chen, Yi-Chun Ke, Chiung-Chyi Shen, Yi-Chin Yang, Chang-Ming Tang, Chun-An Yeh, Hsien-Hsu Hsieh, and Shan-hui Hsu. 2021. "Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells" Nanomaterials 11, no. 8: 2046. https://doi.org/10.3390/nano11082046
APA StyleHung, H. -S., Kung, M. -L., Chen, F. -C., Ke, Y. -C., Shen, C. -C., Yang, Y. -C., Tang, C. -M., Yeh, C. -A., Hsieh, H. -H., & Hsu, S. -h. (2021). Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials, 11(8), 2046. https://doi.org/10.3390/nano11082046