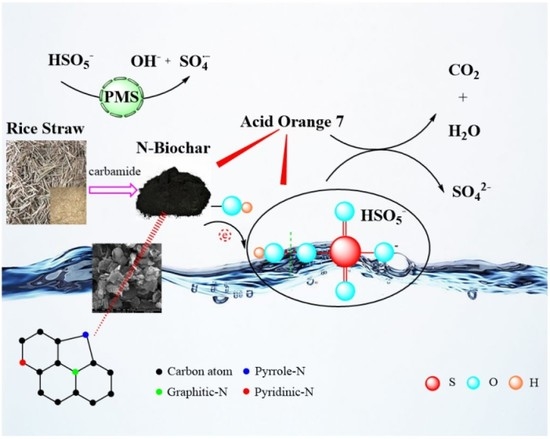
N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Methods
2.2.1. Catalyst Preparation
2.2.2. Characterization Methods
2.2.3. Catalytic Degradation Experiment
2.2.4. Analytical Methods
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Influencing Factors of AO7 Degradation
3.3. ROS Analysis and the Mechanism of Activation
3.4. The PMS-Activating Efficiency of Different Catalysts
3.5. Repeated Use of Catalysts
3.6. Catalyst Applicability Test
3.7. Activation Characteristics of Different Nitrogen-Doped Straw Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.Q.; Li, D.; Wen, Q.X. Investigation of hydrolysis acidification process during anaerobic treatment of coal gasification wastewater (CGW): Evolution of dissolved organic matter and biotoxicity. Sci. Total. Environ. 2020, 723, 137995. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yao, H.Y.; Xu, W.; Liu, G.B.; Wang, X.B.; Tu, Y.; Shi, P.; Yu, N.Y.; Li, A.M.; Wei, S. Suspect screening and risk assessment of pollutants in the wastewater from a chemical industry park in China. Environ. Pollut. 2020, 263, 114493. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Liu, H.X.; Yang, S.G.; Wang, L.S.; Wang, Z.Y. Acute toxicity of benzophenone-type UV filters for Photobacterium phosphoreum and Daphnia magna: QSAR analysis, interspecies relationship and integrated assessment. Chemosphere 2015, 135, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, P.; Liu, H.X.; Yang, S.G.; Wang, L.S.; Wang, Z.Y. Hepatic oxidative stress biomarker responses in freshwater fish Carassius auratus exposed to four commonly benzophenone UV filters. Ecotox. Environ. Saf. 2015, 119, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mercado, L.F.; Lalander, C.; Berger, C.; Dalahmeh, S.S. Potential of Biochar Filters for Onsite Wastewater Treatment: Effects of Biochar Type, Physical Properties and Operating Conditions. Water 2018, 10, 1835. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.Y.; Kim, W.Y.; Son, D.J.; Kim, D.G.; Chang, D.; Chang, S.O.; Sunwoo, Y.; Hong, K.H. Fabrication of tubular-type MF ceramic membrane with enhanced permeability by addition of PMMA in the support and evaluation of physical characteristics for wastewater treatment. Ceram. Int. 2015, 41, 10788–10794. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; He, Q.; Feng, M.B.; Liu, H.X.; Yang, S.G.; Wang, L.S.; Wang, Z.Y. Ozonation of the UV filter benzophenone-4 in aquatic environments: Intermediates and pathways. Chemosphere 2016, 149, 76–83. [Google Scholar] [CrossRef]
- Min, H.C.; Hu, D.X.; Wang, H.C.; Zhao, Y.Y.; Cui, Y.B.; Luo, K.Y.; Zhang, L.F.; Liu, W.Y.; Wu, P.; Ge, H. Electrochemical-assisted hydrolysis/acidification-based processes as a cost-effective and efficient system for pesticide wastewater treatment. Chem. Eng. J. 2020, 397, 125417. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, A.; Babu, D.S.; Scaria, J.; Kumar, M.S. Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation. Chemosphere 2020, 251, 126437. [Google Scholar] [CrossRef]
- Sepulveda-Munoz, C.A.; De Godos, I.; Puyol, D.; Munoz, R. A systematic optimization of piggery wastewater treatment with purple phototrophic bacteria. Chemosphere 2020, 253, 126621. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.M.; Zhang, Y.B.; Wei, Y.J.; Wang, S.; Liu, P.; Jia, Z.H.; Yu, X.M.; Ma, F. Advanced treatment of bio-treated Chinese patent medicine wastewater using ozone/peroxymonosulfate-upflow biological aerated filter. Chem. Eng. J. 2020, 390, 124527. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Wang, H.; Bian, Z.Y. Hybrid electrocatalytic ozonation treatment of high -salinity organic wastewater Ni-Ce/OMC electrodes. Sci. Total. Environ. 2020, 724, 138170. [Google Scholar] [CrossRef]
- Akansha, J.; Nidheesh, P.V.; Gopinath, A.; Anupama, K.V.; Kumar, M.S. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Q.; Wu, M.Y.; Li, R.; Deng, S.H.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Potential of combined advanced oxidation—Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies. Chem. Eng. J. 2020, 389, 123419. [Google Scholar] [CrossRef]
- Goncalves, N.P.F.; Minella, M.; Fabbri, D.; Calza, P.; Malitesta, C.; Mazzotta, E.; Prevot, A.B. Humic acid coated magnetic particles as highly efficient heterogeneous photo-Fenton materials for wastewater treatments. Chem. Eng. J. 2020, 390, 124619. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Madzivire, G.; Leslie, P. A Review of Combined Advanced Oxidation Technologies for the Removal of Organic Pollutants from Water. Water Air Soil. Poll. 2014, 225, 2102. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.H.; Li, C.; Stewart, B.A.; Liu, L.; Zhang, M.; Yang, M.Y.; Lin, K.F. Enhanced thermal activation of peroxymonosulfate by activated carbon for efficient removal of perfluorooctanoic acid. Chem. Eng. J. 2020, 399, 125722. [Google Scholar] [CrossRef]
- Noorisepehr, M.; Ghadirinejad, K.; Kakavandi, B.; Esfahani, A.R.; Asadi, A. Photo-assisted catalytic degradation of acetaminophen using peroxymonosulfate decomposed by magnetic carbon heterojunction catalyst. Chemosphere 2019, 232, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Srivastava, V.; Ambat, I.; Safaei, Z.; Sillanpaa, M. Degradation of Ibuprofen by UV-LED/catalytic advanced oxidation process. J. Water Process Eng. 2019, 31, 100808. [Google Scholar] [CrossRef]
- Lin, C.H.; Shi, D.J.; Wu, Z.T.; Zhang, L.F.; Zhai, Z.C.; Fang, Y.S.; Sun, P.; Han, R.R.; Wu, J.Q.; Liu, H. CoMn2O4 catalyst prepared using the sol-gel method for the activation of peroxymonosulfate and degradation of UV filter 2-phenylbenzimidazole-5-sulfonic acid (PBSA). Nanomaterials 2019, 9, 774. [Google Scholar] [CrossRef] [Green Version]
- Kiani, R.; Mirzaei, F.; Ghanbari, F.; Feizi, R.; Mehdipour, F. Real textile wastewater treatment by a sulfate radicals-Advanced Oxidation Process: Peroxydisulfate decomposition using copper oxide (CuO) supported onto activated carbon. J. Water Process Eng. 2020, 38, 101623. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, P.; Huo, X.W.; Liu, Y.; Liu, Y.X.; Zhang, Y.L. Enhancement of bisphenol A degradation by accelerating the Fe(III)/Fe(II) cycle in graphene oxide modified Fe(III)/peroxymonosulfate system under visible light irradiation. J. Colloid. Interf. Sci. 2020, 580, 540–549. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, D.Y.; Chen, W.Q.; Li, H.; Zhang, S.F.; Liang, C. Covalent organic framework-functionalized magnetic CuFe2O4/Ag nanoparticles for the reduction of 4-Nitrophenol. Nanomaterials 2020, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, W.W.; Wu, D.C.; Tian, T.; Wu, J.F.; Dong, Z.M.; Zhao, G.C. New insight into the mechanism of peroxymonosulfate activation by nanoscaled lead-based spinel for organic matters degradation: A singlet oxygen-dominated oxidation process. J. Colloid. Interf. Sci. 2020, 572, 318–327. [Google Scholar] [CrossRef]
- Tian, X.; Xiao, L. FeOx/MnOy modified oxidized carbon nanotubes as peroxymonosulfate activator for organic pollutants degradation. J. Colloid. Interf. Sci. 2020, 580, 803–813. [Google Scholar] [CrossRef]
- Wang, F.; Wu, C.W.; Li, Q.B. Treatment of refractory organics in strongly alkaline dinitrodiazophenol wastewater with microwave irradiation-activated persulfate. Chemosphere 2020, 254, 126773. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Li, Y.L.; He, J.Y.; Fang, X.; Hong, P.D.; Nie, M.X.; Yang, W.; Xie, C.; Wu, Z.J.; Zhang, K.S. Nano-hybrids of needle-like MnO2 on graphene oxide coupled with peroxymonosulfate for enhanced degradation of norfloxacin: A comparative study and probable degradation pathway. J. Colloid. Interf. Sci. 2020, 562, 1–11. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, C.H.; Qi, J.W.; Yan, Y.B.; Zhang, M.; Yan, X.; Sun, X.Y.; Wang, L.J.; Li, J.S. Spiderweb-like Fe-Co prussian blue analogue nanofibers as efficient catalyst for Bisphenol-A degradation by activating peroxymonosulfate. Nanomaterials 2019, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Zu, J.N.; Yang, L.H.; Shu, X.Q.; Guan, J.D.; Deng, Y.C.; Gong, D.X.; Ding, C.X.; Zhong, M.E. Cobalt (0/II) incorporated N-doped porous carbon as effective heterogeneous peroxymonosulfate catalyst for quinclorac degradation. J. Colloid. Interf. Sci. 2020, 563, 197–206. [Google Scholar] [CrossRef]
- Zuo, S.Y.; Xia, D.S.; Guan, Z.Y.; Yang, F.; Zan, J.; Xu, H.M.; Huang, M.Z.; Li, D.Y. Magnetite/graphite carbon nitride composite for peroxymonosulfate non-radical activation. Colloid. Surface. A 2020, 611, 125895. [Google Scholar] [CrossRef]
- Ge, Y.L.; Zhang, Y.F.; Yang, Y.; Xie, S.; Liu, Y.; Maruyama, T.; Deng, Z.Y.; Zhao, X.L. Enhanced adsorption and catalytic degradation of organic dyes by nanometer iron oxide anchored to single-wall carbon nanotubes. Appl. Surf. Sci. 2019, 488, 813–826. [Google Scholar] [CrossRef]
- Yao, Y.J.; Hu, Y.; Hu, H.H.; Chen, L.W.; Yu, M.J.; Gao, M.X.; Wang, S.B. Metal-free catalysts of graphitic carbon nitride-covalent organic frameworks for efficient pollutant destruction in water. J. Colloid. Interf. Sci. 2019, 554, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Du, M.S.; Chen, K.P.; Lin, Y.P. Degradation of ibuprofen and acetylsulfamethoxazole by multi-walled carbon nanotube catalytic ozonation: Surface properties, kinetics and modeling. Environ. Sci.-Wat. Res. 2019, 5, 1758–1768. [Google Scholar] [CrossRef]
- Sadeghi, M.; Mehdinejad, M.H.; Mengelizadeh, N.; Mandavi, Y.; Pourzamani, H.; Hajizadeh, Y.; Zare, M.R. Degradation of diclofenac by heterogeneous electro-Fenton process using magnetic single-walled carbon nanotubes as a catalyst. J. Water Process Eng. 2019, 31, 100852. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Lee, G.; Kim, S.; Park, C.M.; Jang, M.; Her, N.; Han, J.; Kim, D.H.; Yoon, Y. Sonocatalytic degradation of carbamazepine and diclofenac in the presence of graphene oxides in aqueous solution. Chemosphere 2018, 205, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Tri, N.L.M.; Kim, H.H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process Eng. 2019, 33, 101037. [Google Scholar] [CrossRef]
- Khatibi, E.S.; Haghighi, M.; Mahboob, S. Efficient surface design of reduced graphene oxide, carbon nanotube and carbon active with cupper nanocrystals for enhanced simulated-solar-light photocatalytic degradation of acid orange in water. Appl. Surf. Sci. 2016, 465, 937–949. [Google Scholar] [CrossRef]
- Xiao, R.; Tobin, J.M.; Zha, M.Q.; Hou, Y.L.; He, J.; Vilela, F.; Xu, Z.T. A nanoporous graphene analog for superfast heavy metal removal and continuous-flow visible-light photoredox catalysis. J. Mater. Chem. A 2017, 5, 20180–20187. [Google Scholar] [CrossRef]
- Zhang, X.P.; Liu, D.; Yang, L.; Zhou, L.M.; You, T.Y. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A 2015, 3, 10031–10037. [Google Scholar] [CrossRef]
- Jarrais, B.; Guedes, A.; Freire, C. Heteroatom-Doped Carbon Nanomaterials as Metal-Free Catalysts for the Reduction of 4-Nitrophenol. ChemistrySelect 2018, 3, 1737–1748. [Google Scholar] [CrossRef]
- Yin, R.L.; Guo, W.Q.; Du, J.S.; Zhou, X.J.; Zheng, H.S.; Wu, Q.L.; Chang, J.S.; Ren, N.Q. Heteroatoms doped graphene for catalytic ozonation of sulfamethoxazole by metal-free catalysis: Performances and mechanisms. Chem. Eng. J. 2017, 317, 632–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Yu, Y.B.; Zhou, Y.L.; Hong, J.M. Catalytic performance and mechanism of graphene electrode doped with S and N heteroatoms for N-(4-hydroxyphenyl) ethanamide electrochemical Check tor degradation. J. Hazard. Mater. 2019, 368, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, H.; Feng, M.B.; Zhai, Z.C.; Fang, Y.S.; Zhang, X.S.; Sharma, V.K. Strategic combination of N-doped grapheme and g-C3N4: Efficient catalytic peroxymonosulfate-based oxidation of organic pollutants by nonradical-dominated processes. Appl. Catal. B Environ. 2020, 272, 119005. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Tan, X.F.; Wu, H.P.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.Y.; Chen, Q. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Wang, J.J.; Yu, J.F.; Feng, H.P.; Lu, Y.; Chen, Y.; Liu, Y.N.; Wang, J.J.; Xie, Q.Q. Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil: Active sites and electron transfer mechanism. Colloid. Surface. A 2020, 599, 124904. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Zhai, Z.C.; Zhang, X.S.; Fang, Y.S.; Tan, J.; Wu, J.Q. Degradation of UV filter BP-1 with nitrogen-doped industrial grapheme as a metal-free catalyst of peroxymonosulfate activation. Chem. Eng. J. 2019, 356, 262–271. [Google Scholar] [CrossRef]
- Pedrosa, M.; Drazic, G.; Tavares, P.B.; Figueiredo, J.L.; Silva, A.M.T. Metal-free graphene-based catalytic membrane for degradation of organic contaminants by persulfate activation. Chem. Eng. J. 2019, 369, 223–232. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.B.; Liu, H.X.; Yang, S.G.; Wang, L.S.; Wang, Z.Y. Nitrogen and sulfurco-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfateradicals. Appl. Catal. B Environ. 2016, 187, 1–10. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.B.; Guo, L.; Zhai, Z.C.; Fang, Y.S.; Zhang, X.S.; Sharma, V.K. Nitrogen-sulfur co-doped industrial grapheme as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Du, L.; Xu, W.H.; Liu, S.B.; Li, X.; Huang, D.L.; Tan, X.F.; Liu, Y.G. Activation of persulfate by graphitized biochar for sulfamethoxazole removal: The roles of graphitic carbon structure and carbonyl group. J. Colloid. Interf. Sci. 2020, 577, 419–430. [Google Scholar] [CrossRef]
- Liu, Y.L.; Huang, J.F.; Xu, H.J.; Zhang, Y.L.; Hu, T.; Chen, W.Z.; Hu, H.J.; Wu, J.H.; Li, Y.T.; Jiang, G.B. A magnetic macro-porous biochar sphere as vehicle for the activation and removal of heavy metals from contaminated agricultural soil. Chem. Eng. J. 2020, 390, 124638. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.Y.; Chen, J.J.; Zou, W.X.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 123539. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, S.S.; Song, J.P.; Zhao, Y.; Yang, F. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J. Hazard. Mater. 2020, 389, 122115. [Google Scholar] [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biot. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Kim, D.G.; Ko, S.O. Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, K.; Ma, S.L.; Dang, B.J.; Li, Y.; Fu, H.C.; Du, J.; Meng, Q.X. Activation of peroxymonosulfate by iron-biochar composites: Comparison of nanoscale Fe with single-atom Fe. J. Colloid. Interf. Sci. 2021, 582, 598–609. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.J. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.X.; Liu, P.H.; Bai, X.; Du, X.Y.; Jin, P.K.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Zaeni, J.R.J.; Lim, J.W.; Wang, Z.H.; Ding, D.H.; Chua, Y.S.; Ng, S.L.; Oh, W.D. In situ nitrogen functionalization of biochar via one-pot synthesis for catalytic peroxymonosulfate activation: Characteristics and performance studies. Sep. Purif. Technol. 2020, 241, 116702. [Google Scholar] [CrossRef]
- Rao, L.J.; Yang, Y.F.; Chen, L.K.; Liu, X.D.; Chen, H.X.; Yao, Y.Y.; Wang, W.T. Highly efficient removal of organic pollutants via a green catalytic oxidation system based on sodium metaborate and peroxymonosulfate. Chemosphere 2020, 238, 124687. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Zhou, P.J.; Zhang, J.Y.; Zhu, L.L. Catalytic degradation of Acid Orange 7 in water by persulfate activated with CuFe2O4@RSDBC. Mater. Res. Express. 2020, 7, 016529. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Fang, Y.; Sun, P.; Xie, K.; Zhai, Z.; Liu, H.; Liu, H. N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7. Nanomaterials 2021, 11, 2288. https://doi.org/10.3390/nano11092288
Han R, Fang Y, Sun P, Xie K, Zhai Z, Liu H, Liu H. N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7. Nanomaterials. 2021; 11(9):2288. https://doi.org/10.3390/nano11092288
Chicago/Turabian StyleHan, Ruirui, Yingsen Fang, Ping Sun, Kai Xie, Zhicai Zhai, Hongxia Liu, and Hui Liu. 2021. "N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7" Nanomaterials 11, no. 9: 2288. https://doi.org/10.3390/nano11092288
APA StyleHan, R., Fang, Y., Sun, P., Xie, K., Zhai, Z., Liu, H., & Liu, H. (2021). N-Doped Biochar as a New Metal-Free Activator of Peroxymonosulfate for Singlet Oxygen-Dominated Catalytic Degradation of Acid Orange 7. Nanomaterials, 11(9), 2288. https://doi.org/10.3390/nano11092288