3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability
Abstract
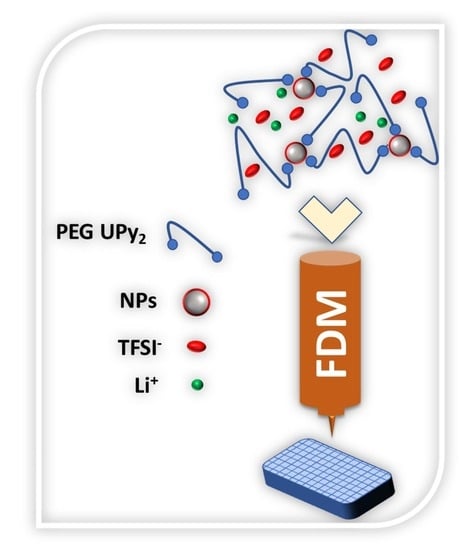
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
3. Results and Discussions
3.1. Modification of PEG Polymers
3.2. Modification of Nanoparticles
3.2.1. Modification of LUDOX® SM SiO2 NPs by Using Ionic Liquid Groups
3.2.2. Modification of Silica Nanopowder
3.2.3. Characterization of Modified Nanoparticles
3.3. 3D Printability of Composite Electrolytes via Rheology
3.3.1. Rheology Measurements of PEG 8000 B2 without Nanofillers
3.3.2. Rheology Measurements of PEG 1500 UPy and PEG 8000 B2 with Nanofillers
3.4. Conductivity
3.5. 3D Printing of Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Rollo-Walker, G.; Malic, N.; Wang, X.; Chiefari, J.; Forsyth, M. Development and Progression of Polymer Electrolytes for Batteries: Influence of Structure and Chemistry. Polymers 2021, 13, 4127. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, G.G.; Armand, M.; Passerini, S. Lithium Polymer Electrolytes and Batteries. In Prospects for Li-ion Batteries and Emerging Energy Electrochemical Systems; World Scientific: Singapore, 2018; pp. 319–364. [Google Scholar]
- Boaretto, N.; Meabe, L.; Martinez-Ibañez, M.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Rechargeable Batteries: From Nanocomposite to Nanohybrid. J. Electrochem. Soc. 2020, 167, 070524. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Keller, M.; Varzi, A.; Passerini, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 2018, 392, 206–225. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; So, S.; Hur, J. Inorganic Fillers in Composite Gel Polymer Electrolytes for High-Performance Lithium and Non-Lithium Polymer Batteries. Nanomaterials 2021, 11, 614. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.C.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Nematdoust, S.; Najjar, R.; Bresser, D.; Passerini, S. Understanding the Role of Nanoparticles in PEO-Based Hybrid Polymer Electrolytes for Solid-State Lithium–Polymer Batteries. J. Phys. Chem. C 2020, 124, 27907–27915. [Google Scholar] [CrossRef]
- Lu, Y.; Korf, K.; Kambe, Y.; Tu, Z.; Archer, L.A. Ionic-Liquid–Nanoparticle Hybrid Electrolytes: Applications in Lithium Metal Batteries. Angew. Chem. Int. Ed. 2014, 53, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Marinow, A.; Rupp, H.; Binder*, W.H. Materialien für die “Ewigkeit”: Selbstheilende Polymere–auch rezyklierbar! Chemie in unserer Zeit 2021. [Google Scholar] [CrossRef]
- Mai, W.; Yu, Q.; Han, C.; Kang, F.; Li, B. Self-Healing Materials for Energy-Storage Devices. Adv. Funct. Mater. 2020, 30, 1909912. [Google Scholar] [CrossRef]
- Campanella, A.; Döhler, D.; Binder, W.H. Self-Healing in Supramolecular Polymers. Macromol. Rapid Commun. 2018, 39, 1700739. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W. Supramolecular Polymers–we’ve Come Full Circle. Isr. J. Chem. 2020, 60, 33–47. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Zhang, T.; Wang, X.; Gao, Y.; Fang, Q. Self-healing strategy for Si nanoparticles towards practical application as anode materials for Li-ion batteries. Electrochem. Commun. 2018, 87, 22–26. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Small 2018, 14, 1801189. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Review—Emerging Trends in the Design of Electrolytes for Lithium and Post-Lithium Batteries. J. Electrochem. Soc. 2020, 167, 050508. [Google Scholar] [CrossRef]
- Rupp, H.; Döhler, D.; Hilgeroth, P.; Mahmood, N.; Beiner, M.; Binder, W.H. 3D Printing of Supramolecular Polymers: Impact of Nanoparticles and Phase Separation on Printability. Macromol. Rapid Commun. 2019, 40, 1900467. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Cao, Y.; Chu, Y.; Liu, M.; Snyder, K.; MacKenzie, D.; Cao, C. Additive Manufacturing of Batteries. Adv. Funct. Mater. 2020, 30, 1906244. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Hao, J.; Wallace, G.G.; Beirne, S.; Chen, J. 3D-Printed Wearable Electrochemical Energy Devices. Adv. Funct. Mater. 2022, 32, 2103092. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevée, J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Shi, H.; Yao, F.; Liang, S.; Xu, Z.; Pei, X.; Wang, S.; Hu, Y. 3D printing of advanced lithium batteries: A designing strategy of electrode/electrolyte architectures. J. Mater. Chem. A 2021, 9, 25237–25257. [Google Scholar] [CrossRef]
- Cheng, M.; Deivanayagam, R.; Shahbazian-Yassar, R. 3D Printing of Electrochemical Energy Storage Devices: A Review of Printing Techniques and Electrode/Electrolyte Architectures. Batter. Supercaps 2020, 3, 130–146. [Google Scholar] [CrossRef]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly(Ethylene Oxide)−LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [Google Scholar] [CrossRef]
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing Strategies for Solid Electrolyte in Batteries. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Rupp, H.; Bhandary, R.; Kulkarni, A.; Binder, W.H. Printable Electrolytes: Tuning 3D-Printing by Multiple Hydrogen Bonds and Added Inorganic Lithium-Salts. Adv. Mater. Technol. 2022, 2200088. [Google Scholar] [CrossRef]
- Folmer, B.J.B.; Sijbesma, R.P.; Versteegen, R.M.; van der Rijt, J.A.J.; Meijer, E.W. Supramolecular Polymer Materials: Chain Extension of Telechelic Polymers Using a Reactive Hydrogen-Bonding Synthon. Adv. Mater. 2000, 12, 874–878. [Google Scholar] [CrossRef]
- Mollet, B.B.; Comellas-Aragonès, M.; Spiering, A.J.H.; Söntjens, S.H.M.; Meijer, E.W.; Dankers, P.Y.W. A modular approach to easily processable supramolecular bilayered scaffolds with tailorable properties. J. Mater. Chem. B. 2014, 2, 2483–2493. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hilgeroth, P.; Hasan, N.; Ströhl, D.; Kressler, J.; Binder, W.H. Comparing C2=O and C2=S Barbiturates: Different Hydrogen-Bonding Patterns of Thiobarbiturates in Solution and the Solid State. Int. J. Mol. Sci. 2021, 22, 12679. [Google Scholar] [CrossRef]
- Herbst, F.; Seiffert, S.; Binder, W.H. Dynamic supramolecular poly(isobutylene)s for self-healing materials. Polym. Chem. 2012, 3, 3084–3092. [Google Scholar] [CrossRef]
- Yan, T.; Schröter, K.; Herbst, F.; Binder, W.H.; Thurn-Albrecht, T. Unveiling the molecular mechanism of self-healing in a telechelic, supramolecular polymer network. Sci. Rep. 2016, 6, 32356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Schröter, K.; Herbst, F.; Binder, W.H.; Thurn-Albrecht, T. What Controls the Structure and the Linear and Nonlinear Rheological Properties of Dense, Dynamic Supramolecular Polymer Networks? Macromolecules 2017, 50, 2973–2985. [Google Scholar] [CrossRef] [Green Version]
- Moganty, S.S.; Srivastava, S.; Lu, Y.; Schaefer, J.L.; Rizvi, S.A.; Archer, L.A. Ionic Liquid-Tethered Nanoparticle Suspensions: A Novel Class of Ionogels. Chem. Mater. 2012, 24, 1386–1392. [Google Scholar] [CrossRef]
- Rey, I.; Johansson, P.; Lindgren, J.; Lassègues, J.C.; Grondin, J.; Servant, L. Spectroscopic and Theoretical Study of (CF3SO2)2N- (TFSI-) and (CF3SO2)2NH (HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258. [Google Scholar] [CrossRef]
- Kim, K.; Kuhn, L.; Alabugin, I.V.; Hallinan, D.T. Lithium Salt Dissociation in Diblock Copolymer Electrolyte Using Fourier Transform Infrared Spectroscopy. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Rupp, H.; Binder, W.H. 3D Printing of Solvent-Free Supramolecular Polymers. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential Scanning Calorimetry (DSC). In Thermal Analysis of Polymers; Wiley: Hoboken, HJ, USA, 2009; pp. 7–239. [Google Scholar]
- Marzantowicz, M.; Krok, F.; Dygas, J.R.; Florjańczyk, Z.; Zygadło-Monikowska, E. The influence of phase segregation on properties of semicrystalline PEO:LiTFSI electrolytes. Solid State Ion. 2008, 179, 1670–1678. [Google Scholar] [CrossRef]
- Rupp, H.; Binder, W.H. Multicomponent Stress-Sensing Composites Fabricated by 3D-Printing Methodologies. Macromol. Rapid Commun. 2021, 42, 2000450. [Google Scholar] [CrossRef]
- Wen, S.J.; Richardson, T.J.; Ghantous, D.I.; Striebel, K.A.; Ross, P.N.; Cairns, E.J. FTIR characterization of PEO + LiN(CF3SO2)2 electrolytes. J. Electroanal. Chem. 1996, 408, 113–118. [Google Scholar] [CrossRef]











| Entry | Polymer | Molar EO/Li | Nanoparticles | (wt%) Nanoparticles |
|---|---|---|---|---|
| 1 | PEG 1500 UPy2 | 5:1 | - | - |
| 2 | PEG 1500 UPy2 | 5:1 | NP–IL | 5 |
| 3 | PEG 1500 UPy2 | 5:1 | NP–IL | 10 |
| 4 | PEG 1500 UPy2 | 5:1 | NP–IL | 15 |
| 5 | PEG 1500 UPy2 | 5:1 | NP–OH | 5 |
| 6 | PEG 1500 UPy2 | 5:1 | NP–OH | 10 |
| 7 | PEG 1500 UPy2 | 5:1 | NP–OH | 15 |
| 8 | PEG 8000 B2 | 5:1 | - | - |
| 9 | PEG 8000 B2 | 10:1 | - | - |
| 10 | PEG 8000 B2 | 20:1 | - | - |
| 11 | PEG 8000 B2 | 5:1 | NP–alk | 5 |
| 12 | PEG 8000 B2 | 5:1 | NP–alk | 10 |
| 13 | PEG 8000 B2 | 5:1 | NP–alk | 15 |
| 14 | PEG 8000 B2 | 5:1 | NP–IL | 10 |
| 15 | PEG 8000 B2 | - | NP–alk | 5 |
| 16 | PEG 8000 B2 | - | NP–alk | 10 |
| 17 | PEG 8000 B2 | - | NP–alk | 15 |
| 18 | PEG 8000 B2 | - | NP–OH | 5 |
| 19 | PEG 8000 B2 | - | NP–OH | 10 |
| 20 | PEG 8000 B2 | - | NP–OH | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katcharava, Z.; Marinow, A.; Bhandary, R.; Binder, W.H. 3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability. Nanomaterials 2022, 12, 1859. https://doi.org/10.3390/nano12111859
Katcharava Z, Marinow A, Bhandary R, Binder WH. 3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability. Nanomaterials. 2022; 12(11):1859. https://doi.org/10.3390/nano12111859
Chicago/Turabian StyleKatcharava, Zviadi, Anja Marinow, Rajesh Bhandary, and Wolfgang H. Binder. 2022. "3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability" Nanomaterials 12, no. 11: 1859. https://doi.org/10.3390/nano12111859
APA StyleKatcharava, Z., Marinow, A., Bhandary, R., & Binder, W. H. (2022). 3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability. Nanomaterials, 12(11), 1859. https://doi.org/10.3390/nano12111859