Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
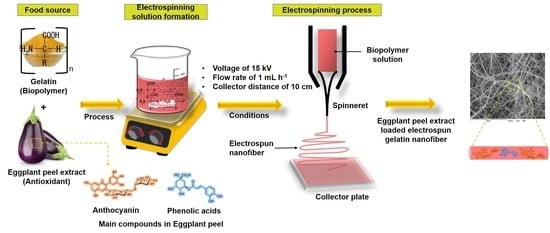
2.2. Preparation of Eggplant Peel Extract
2.3. Preparation of Solutions
2.4. Electrospinning Process
2.5. Characterization of Electrospun Nanofiber
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Transmission Electronic Microscopy (TEM)
2.5.3. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.6. Encapsulation Efficiency (EE)
2.7. In Vitro Release
2.8. Statistic Analysis
3. Results and Discussion
3.1. Morphology of Eggplant Peel Extract Loaded Electrospun Gelatin Nanofibers
3.1.1. Morphology by SEM
3.1.2. Morphology by TEM
3.2. Fiber Size Distribution
3.3. Interaction of Eggplant Peel Extract-Loaded Electrospun Gelatin Nanofiber
3.4. Encapsulation Efficiency
3.5. In Vitro Release of Eggplant Peel Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and Cytotoxicity Effects of Synthesized Silver Nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Carvajal-Millan, E.; López-Ahumada, G.A.; Castro-Enriquez, D.D.; Barreras-Urbina, C.G.; Rodríguez-Felix, F. Prolamins from cereal by-products: Classification, extraction, characterization and its applications in micro- and nanofabrication. Trends Food Sci. Technol. 2019, 90, 111–132. [Google Scholar] [CrossRef]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Philippi, K.; Tsamandouras, N.; Grigorakis, S.; Makris, D.P. Ultrasound-Assisted Green Extraction of Eggplant Peel (Solanum melongena) Polyphenols Using Aqueous Mixtures of Glycerol and Ethanol: Optimisation and Kinetics. Environ. Process. 2016, 3, 369–386. [Google Scholar] [CrossRef]
- Gurbuz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A. Introduction: The Importance of Eggplant. In The Eggplant Genome; Springer: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochemistry 2016, 31, 637–646. [Google Scholar] [CrossRef]
- Braga, P.C.; Scalzo, R.L.; Sasso, M.D.; Lattuada, N.; Greco, V.; Fibiani, M. Characterization and antioxidant activity of semi-purified extracts and pure delphinidin-glycosides from eggplant peel (Solanum melongena L.). J. Funct. Foods 2016, 20, 411–421. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Total Monomeric Anthocyanin, Total Phenolic Content and Antioxidant Activity of Extracts from Eggplant (Solanum melongena L.) Peel Using Ultrasonic Treatments. J. Food Process Eng. 2017, 40, e12312. [Google Scholar] [CrossRef]
- Todaro, A.; Cimino, F.; Rapisarda, P.; Catalano, A.; Barbagallo, R.; Spagna, G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009, 114, 434–439. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Scalzo, R.L.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wang, Q.; Sun, R.; Li, T.; Xia, N.; Xia, Q. A novel solid self-emulsifying delivery system (SEDS) for the encapsulation of linseed oil and quercetin: Preparation and evaluation. J. Food Eng. 2018, 226, 22–30. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; López-Ahumada, G.A.; Carvajal-Millan, E.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Preparation and characterization of quercetin-loaded zein nanoparticles by electrospraying and study of in vitro bioavailability. J. Food Sci. 2019, 84, 2883–2897. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Hii, Y.S.; Jeevanandam, J.; San Chan, Y.; Danquah, M.K. Nanoencapsulation of Phytochemicals and in-vitro Applications. In Phytochemistry: An In-Silico and In-Vitro Update; Springer: Singapore, 2019; pp. 315–330. [Google Scholar] [CrossRef]
- Horuz, T.; Belibağli, K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019, 99, 759–766. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Deladino, L.; Navarro, A.S.; Amalvy, J.I.; Martino, M.N. Yerba Mate Extract Encapsulation with Alginate and Chitosan Systems: Interactions between Active Compound Encapsulation Polymers. J. Encapsulation Adsorpt. Sci. 2011, 1, 80–87. [Google Scholar] [CrossRef] [Green Version]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramirez-Pimentel, J.G.; Vargas-Torres, A.; Hernández-Fuentes, A.D. Physicochemical and antioxidant properties of gelatin-based films containing oily tomato extract (Solanum lycopersicum L.). CyTA J. Food 2019, 17, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers. Int. J. Biol. Macromol. 2019, 124, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A. A new design for obtaining of white zein micro- and nanoparticles powder: Antisolvent-dialysis method. Food Sci. Biotechnol. 2020, 29, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. Nanoencapsulation: Techniques and Developments for Food Applications. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–61. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2021, 12, 485–499. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod. Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-dissolving electrospun gelatin nanofibers encapsulating ciprofloxacin/cyclodextrin inclusion complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef]
- Stoll, L.; Costa, T.M.H.; Jablonski, A.; Flores, S.; Rios, A.D.O. Microencapsulation of Anthocyanins with Different Wall Materials and Its Application in Active Biodegradable Films. Food Bioprocess Technol. 2016, 9, 172–181. [Google Scholar] [CrossRef]
- Hani, N.M.; Torkamani, A.E.; Azarian, M.H.; Mahmood, K.W.; Ngalim, S.H. Characterisation of electrospun gelatine nanofibres encapsulated with Moringa oleifera bioactive extract. J. Sci. Food Agric. 2017, 97, 3348–3358. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; de Medeiros, P.M.; Prudencio, E.S.; Müller, C.; Amboni, R.D.D.M.C. Encapsulation of aqueous leaf extract of Stevia rebaudiana Bertoni with sodium alginate and its impact on phenolic content. Food Biosci. 2016, 13, 32–40. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Processing Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Davudian, S.H.; Pourmohammad, S.; Hamishehkar, H.; Roufegarinejad, L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr. Polym. 2019, 216, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple peel extract incorporated poly (vinyl alcohol)-corn starch film for active food packaging: Preparation, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Pereira, S.A.; Souza, C.P.; Moraes, L.; Fontes-Sant’Ana, G.C.; Amaral, P.F. Polymers as Encapsulating Agents and Delivery Vehicles of Enzymes. Polymers 2021, 13, 4061. [Google Scholar] [CrossRef] [PubMed]
- Trilokia, M.; Bandral, J.D.; Chib, A.; Choudhary, P. Microencapsulation for food: An overview. Pharma Innov. J. 2022, 11, 1174–1180. [Google Scholar]
- Meng, J.; Lin, X.; Zhou, J.; Zhang, R.; Chen, Y.; Long, X.; Shang, R.; Luo, X. Preparation of tannin-immobilized gelatin/PVA nanofiber band for extraction of uranium (VI) from simulated seawater. Ecotoxicol. Environ. Saf. 2019, 170, 9–17. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella-Osuna, D.E.; Tapia-Hernández, J.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials 2022, 12, 2303. https://doi.org/10.3390/nano12132303
Estrella-Osuna DE, Tapia-Hernández JA, Ruíz-Cruz S, Márquez-Ríos E, Ornelas-Paz JdJ, Del-Toro-Sánchez CL, Ocaño-Higuera VM, Rodríguez-Félix F, Estrada-Alvarado MI, Cira-Chávez LA. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials. 2022; 12(13):2303. https://doi.org/10.3390/nano12132303
Chicago/Turabian StyleEstrella-Osuna, Danya Elizabeth, José Agustín Tapia-Hernández, Saúl Ruíz-Cruz, Enrique Márquez-Ríos, José de Jesús Ornelas-Paz, Carmen Lizette Del-Toro-Sánchez, Víctor Manuel Ocaño-Higuera, Francisco Rodríguez-Félix, María Isabel Estrada-Alvarado, and Luis Alberto Cira-Chávez. 2022. "Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release" Nanomaterials 12, no. 13: 2303. https://doi.org/10.3390/nano12132303
APA StyleEstrella-Osuna, D. E., Tapia-Hernández, J. A., Ruíz-Cruz, S., Márquez-Ríos, E., Ornelas-Paz, J. d. J., Del-Toro-Sánchez, C. L., Ocaño-Higuera, V. M., Rodríguez-Félix, F., Estrada-Alvarado, M. I., & Cira-Chávez, L. A. (2022). Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials, 12(13), 2303. https://doi.org/10.3390/nano12132303