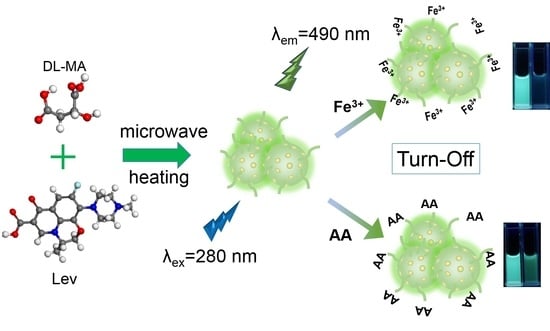
One-Step Synthesis of Nitrogen/Fluorine Co-Doped Carbon Dots for Use in Ferric Ions and Ascorbic Acid Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NFCDs
2.3. Characterization of NFCDs
2.4. Stability of NFCDs Solution
2.5. Fluorescence Quenching Study of Fe3+ and AA
3. Results and Discussion
3.1. Morphology and Structure Characterization of NFCDs
3.2. Optical Property Characterization of NFCDs
3.3. Stability of NFCDs
3.4. Sensitivity and Selectivity Study of Fe3+ Ion
3.5. Sensitivity and Selectivity Study of AA
3.6. Possible Quenching Mechanism of Fe3+ and AA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreani, A.S.; Kunarti, E.S.; Hashimoto, T.; Hayashita, T.; Santosa, S.J. Fast and selective colorimetric detection of Fe3+ based on gold nanoparticles capped with ortho-hydroxybenzoic acid. J. Environ. Chem. Eng. 2021, 9, 105962–105968. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Li, D.C.; Cheng, B.H.; Jiang, H. Highly stable and selective measurement of Fe3+ ions under environmentally relevant conditions via an excitation-based multiwavelength method using N, S-doped carbon dots. Environ. Res. 2019, 170, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lesani, P.; Ardekani, S.M.; Dehghani, A.; Hassan, M.; Gomes, V.G. Excitation-independent carbon dot probes for exogenous and endogenous Fe3+ sensing in living cells: Fluorescence lifetime and sensing mechanism. Sens. Actuators B Chem. 2019, 285, 145–155. [Google Scholar] [CrossRef]
- Nagaraj, M.; Ramalingam, S.; Murugan, C.; Aldawood, S.; Jin, J.O.; Choi, I.; Kim, M. Detection of Fe3+ ions in aqueous environment using fluorescent carbon quantum dots synthesized from endosperm of Borassus flabellifer. Environ. Res. 2022, 212, 113273–113280. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, R.; Chen, F.; Yan, G.; Tian, W.; Zhang, J.; Zhang, J. Electrochemical process of early-stage corrosion detection based on N-doped carbon dots with superior Fe3+ responsiveness. J. Colloid Interface Sci. 2022, 606, 567–576. [Google Scholar] [CrossRef]
- Eisenstein, R.S. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Ann. Rev. Nutr. 2000, 20, 627–632. [Google Scholar] [CrossRef]
- Cairo, G.; Pietrangelo, A. Iron regulatory proteins in pathobiology. Biochem. J. 2000, 352, 241–250. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Sotelo Rios, S.E.; Agarwal, V. Simple one step synthesis of dual-emissive heteroatom doped carbon dots for acetone sensing in commercial products and Cr (VI) reduction. Chem. Eng. J. 2021, 414, 128830–128842. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Niu, N. An off-on fluorescent probe based on graphene quantum dots intercalated hydrotalcite for determination of ascorbic acid and phytase. Sens. Actuators B Chem. 2021, 345, 130353–130362. [Google Scholar] [CrossRef]
- Wang, T.; Luo, H.; Jing, X.; Yang, J.; Huo, M.; Wang, Y. Synthesis of Fluorescent Carbon Dots and Their Application in Ascorbic Acid Detection. Molecules 2021, 26, 1246. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.X.; Li, P.; Zhang, W.; Pang, X.; Wang, H.; Tang, B. Fluorescence biosensor for Fe(III) in cells based on Fe(III) catalytze Au-nanocomposites release Au NPs. Sens. Actuators B 2019, 286, 16–21. [Google Scholar] [CrossRef]
- Lin, X.; Xuan, D.L.; Li, F.F.; Liu, C.; Fan, P.F.; Xiao, F.B.; Liang, H.; Yang, S.Y. DNA-AgNCs as a fluorescence turn-off probe for dual functional detection of H2O2 and Fe(II) ions. Spectrochim. Acta A 2020, 229, 117894–117898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xue, L.; Li, G.; Jiang, H. A highly sensitive near-infrared ratiometric fluorescent probe for detecting nitroreductase and cellular imaging. Sens. Actuators B 2016, 222, 419–424. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, B.; Liu, J.L. Nanoscale metal-organic frameworks as highly sensitive luminescent sensors for Fe2+ in aqueous solution and living cells. Chem. Commun. 2014, 50, 9969–9972. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hou, X.; Xu, J.-J.; Chen, H.-Y. Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots. Nanoscale 2016, 8, 8427–8442. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Song, W.Z.; Li, Y.; Wang, L.Y.; Pandey, N.K.; Chudal, L.; Wang, M.; Li, Y.C.; Zhao, L.L.; Yin, W.Z.; et al. The exploration of novel fluorescent copper-cysteamine nanosheets for sequential detection of Fe3+ and dopamine and fabrication of molecular logic circuits. J. Mater. Chem. C 2020, 8, 12935–12942. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, H.; Huang, Y.; Zhang, F.; Liu, H.; Liu, H.; Wang, Z.J.; Li, R. Highly fluorescent nitrogen and boron doped carbon quantum dots for selective and sensitive detection of Fe3+. J. Mater. Chem. B 2021, 9, 4654–4662. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of Heteroatom-Doped Carbon Dots onto Nonpolar Plastics for Antifogging, Antioxidant, and Food Monitoring Applications. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, S.; Wu, S.; Huang, L.; Xu, T.; Xing, X.; Lan, M.; Song, X. A carbon dots-based fluorescent probe for turn-on sensing of ampicillin. Dye. Pigment. 2020, 172, 107846–107853. [Google Scholar] [CrossRef]
- Das, P.; Bose, M.; Das, A.K.; Banerjee, S.; Das, N.C. One-Step Synthesis of Fluorescent Carbon Dots for Bio-Labeling Assay. Macromol. Symp. 2018, 382, 1800077–1800082. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ren, D.; Chai, Y.; Cheng, X.; Mei, J.; Bao, J.; Wei, F.; Xu, G.; Hu, Q.; Cen, Y. Dual-emission carbon dots-based fluorescent probe for ratiometric sensing of Fe(III) and pyrophosphate in biological samples. Sens. Actuators B Chem. 2019, 298, 126829–126834. [Google Scholar] [CrossRef]
- Gong, X.; Lu, W.; Paau, M.C.; Hu, Q.; Wu, X.; Shuang, S.; Dong, C.; Choi, M.M.F. Facile synthesis of nitrogen-doped carbon dots for Fe3+ sensing and cellular imaging. Anal. Chim. Acta. 2015, 861, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Q.; Zhao, X.; Yuan, J.; Zhao, H.; Wang, G.; Li, M. Synthesis of corn straw-based graphene quantum dots (GQDs) and their application in PO43− detection. J. Environ. Chem. Eng. 2022, 10, 107150–107159. [Google Scholar] [CrossRef]
- Shen, C.; Dong, C.; Cheng, L.; Shi, X.; Bi, H. Fluorescent carbon dots from Shewanella oneidensis MR–1 for Hg2+ and tetracycline detection and selective fluorescence imaging of Gram–positive bacteria. J. Environ. Chem. Eng. 2022, 10, 107020–107029. [Google Scholar] [CrossRef]
- Li, C.; Zeng, J.; Guo, D.; Liu, L.; Xiong, L.; Luo, X.; Hu, Z.; Wu, F. Cobalt-Doped Carbon Quantum Dots with Peroxidase-Mimetic Activity for Ascorbic Acid Detection through Both Fluorometric and Colorimetric Methods. ACS Appl. Mater. Interfaces 2021, 13, 49453–49461. [Google Scholar] [CrossRef]
- Xu, M.S.; Dong, C.; Xu, J.H.; Rehman, S.U.; Wang, Q.Y.; Osipov, V.Y.; Jiang, K.; Wang, J.F.; Bi, H. Fluorinated carbon dots/carboxyl methyl cellulose sodium composite with a temperature-sensitive fluorescence/phosphorescence applicable for anti-counterfeiting marking. Carbon 2022, 189, 459–466. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chem. Eng. J. 2022, 430, 133101–133113. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Zheng, J.; Yang, Y.; Liu, X.; Xu, B. N, B-Codoping Induces High-Efficiency Solid-State Fluorescence and Dual Emission of Yellow/Orange Carbon Dots. ACS Sustain. Chem. Eng. 2021, 9, 2224–2236. [Google Scholar] [CrossRef]
- Fu, Q.; Long, C.; Huang, J.; Liu, S.; Qing, T.; Zhang, P.; Feng, B. Highly sensitive B, N co-doped carbon dots for fluorescent and colorimetric dual-mode detection of mercury ions in wastewater. J. Environ. Chem. Eng. 2021, 9, 106882–106890. [Google Scholar] [CrossRef]
- Ge, J.; Shen, Y.; Wang, W.; Li, Y.; Yang, Y. N-doped carbon dots for highly sensitive and selective sensing of copper ion and sulfide anion in lake water. J. Environ. Chem. Eng. 2021, 9, 105081–105088. [Google Scholar] [CrossRef]
- Wang, W.; Peng, J.; Li, F.; Su, B.; Chen, X.; Chen, X. Phosphorus and chlorine co-doped carbon dots with strong photoluminescence as a fluorescent probe for ferric ions. Microchim. Acta 2018, 186, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, W.; Luo, J.; Fan, J.; Wu, Z.-c.; Zhu, H.; Huang, J.; Zhang, X. High-efficient and pH-sensitive orange luminescence from silicon-doped carbon dots for information encryption and bio-imaging. J. Colloid Interface Sci. 2022, 607, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yan, H.; Feng, Y.; Feng, W.; Yuan, L. Multi-excitation and single color emission carbon dots doped with silicon and nitrogen: Synthesis, emission mechanism, Fe3+ probe and cell imaging. Chem. Eng. J. 2019, 373, 963–972. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Li, Y.; Feng, Y.; Feng, W. Room-temperature phosphorescent fluorine-nitrogen co-doped carbon dots: Information encryption and anti-counterfeiting. Carbon 2021, 181, 9–15. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, X.; Xie, K.; Yan, Y.; Ren, B.; Liu, R.; Li, Y.; Li, L. Fluorescent Carbon Dots as Nanosensors for Monitoring and Imaging Fe3+ and [HPO4]2– Ions. ACS Appl. Nano Mater. 2021, 4, 190–197. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, X.; Liu, Y.; Liang, L.; Peng, Y.; Wu, S.; Zhao, Y. N-Doped Carbon Dots as Fluorescent “Turn-Off” Nanosensors for Ascorbic Acid and Fe3+ Detection. ACS Appl. Nano Mater. 2022, 5, 7268–7277. [Google Scholar] [CrossRef]
- Mishra, L.; Behera, R.K.; Mondal, S.; Kumar, S.; Panigrahi, A.; Sarangi, M.K. Interface and doping in carbon dots influence charge transfer and transport. Carbon 2021, 178, 594–605. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, H.; Shen, J.; Wang, C.; Wei, Y. Nitrogen and fluorine co-doped green fluorescence carbon dots as a label-free probe for determination of cytochrome c in serum and temperature sensing. J. Colloid Interface Sci. 2021, 586, 683–691. [Google Scholar] [CrossRef]
- Wu, X.; Xu, M.; Wang, S.; Abbas, K.; Huang, X.; Zhang, R.; Tedesco, A.C.; Bi, H.F. N-Doped carbon dots as efficient Type I photosensitizers for photodynamic therapy. Dalton Trans. 2022, 51, 2296–2303. [Google Scholar] [CrossRef]
- Ding, H.; Xu, J.; Jiang, L.; Dong, C.; Meng, Q.; Rehman, S.u.; Wang, J.; Ge, Z.; Osipov, V.Y.; Bi, H. Fluorine-defects induced solid-state red emission of carbon dots with an excellent thermosensitivity. Chin. Chem. Lett. 2021, 32, 3646–3651. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Yang, Y.; Tang, S.; Li, X.; Xie, F.; Wang, G.; Guo, Q. Synthesis of multi-color fluorine and nitrogen co-doped graphene quantum dots for use in tetracycline detection, colorful solid fluorescent ink, and film. J. Colloid Interface Sci. 2021, 602, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Xu, X.; Liu, Z.; Liu, H.; Lei, B.; Zhuang, J.; Guo, Z.; Liu, Y.; Hu, C. Visible-light excitable thermally activated delayed fluorescence in aqueous solution from F, N-doped carbon dots confined in silica nanoparticles. Chem. Eng. J. 2021, 426, 130728–130736. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Wang, Q.; Luo, K.H. Green Synthesis of Tunable Fluorescent Carbon Quantum Dots from Lignin and Their Application in Anti-Counterfeit Printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef]
- Shen, J.; Xu, B.; Wang, Z.; Zhang, J.; Zhang, W.; Gao, Z.; Wang, X.; Zhu, C.; Meng, X. Aggregation-induced room temperature phosphorescent carbonized polymer dots with wide-range tunable lifetimes for optical multiplexing. J. Mater. Chem. C 2021, 9, 6781–6788. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.; Gao, L.; Xiong, J.; Tan, K. Strategy to Synthesize Tunable Multiemission Carbon Dots and Their Multicolor Visualization Application. ACS Appl. Mater. Interfaces 2021, 13, 33354–33362. [Google Scholar] [CrossRef]
- Tan, J.; Li, Q.; Meng, S.; Li, Y.; Yang, J.; Ye, Y.; Tang, Z.; Qu, S.; Ren, X. Time-Dependent Phosphorescence Colors from Carbon Dots for Advanced Dynamic Information Encryption. Adv. Mater. 2021, 33, 2006781–2006787. [Google Scholar] [CrossRef]
- Long, P.; Feng, Y.Y.; Cao, C.; Li, Y.; Han, J.K.; Li, S.W.; Peng, C.; Li, Z.Y.; Feng, W. Self-Protective Room-Temperature Phosphorescence of Fluorine and Nitrogen Codoped Carbon Dots. Adv. Funct. Mater. 2018, 28, 1800791–1800800. [Google Scholar] [CrossRef]
- Sim, Y.; Kim, S.J.; Janani, G.; Chae, Y.; Surendran, S.; Kim, H.; Yoo, S.; Seok, D.C.; Jung, Y.H.; Jeon, C.; et al. The synergistic effect of nitrogen and fluorine co-doping in graphene quantum dot catalysts for full water splitting and supercapacitor. Appl. Surf. Sci. 2020, 507, 145157–145164. [Google Scholar] [CrossRef]
- Liu, E.; Li, D.; Zhou, X.; Zhou, G.; Xiao, H.; Zhou, D.; Tian, P.; Guo, R.; Qu, S. Highly Emissive Carbon Dots in Solid State and Their Applications in Light-Emitting Devices and Visible Light Communication. ACS Sustain. Chem. Eng. 2019, 7, 9301–9308. [Google Scholar] [CrossRef]
- Wang, B.; Mu, Y.; Zhang, C.; Li, J. Blue photoluminescent carbon nanodots prepared from zeolite as efficient sensors for picric acid detection. Sens. Actuators B Chem. 2017, 253, 911–917. [Google Scholar] [CrossRef]
- Ma, W.; Wang, B.; Yang, Y.; Li, J. Photoluminescent chiral carbon dots derived from glutamine. Chin. Chem. Lett. 2021, 32, 3916–3920. [Google Scholar] [CrossRef]
- Macairan, J.-R.; de Medeiros, T.V.; Gazzetto, M.; Yarur Villanueva, F.; Cannizzo, A.; Naccache, R. Elucidating the mechanism of dual-fluorescence in carbon dots. J. Colloid Interface Sci. 2022, 606, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Arkin, K.; Hao, J.; Zhang, S.; Guan, W.; Wang, L.; Guo, Y.; Shang, Q. Multicolor Carbon Dots Prepared by Single-Factor Control of Graphitization and Surface Oxidation for High-Quality White Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2100688–2100698. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, H.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Dai, S.; Wang, J.; Fang, Z. Synthesis of blue fluorescent carbon dots and their application in detecting mercury and iodine based on “off–on” mode. Luminescence 2021, 36, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kang, D.H. Effect of amino acid-derived nitrogen and/or sulfur doping on the visible-light-driven antimicrobial activity of carbon quantum dots: A comparative study. Chem. Eng. J. 2021, 420, 129990–130003. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, pH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Pang, S.; Liu, S. Dual-emission carbon dots for ratiometric detection of Fe3+ ions and acid phosphatase. Anal. Chim. Acta 2020, 1105, 155–161. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-Doped Carbon Quantum Dots as Fluorometric Probes for Iron Detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Wu, X.; Ma, C.; Luo, S.; Xu, M.; Li, W.; Liu, S. Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 2020, 210, 120649–120657. [Google Scholar] [CrossRef]
- Yue, J.; Li, L.; Cao, L.; Zan, M.; Yang, D.; Wang, Z.; Chang, Z.; Mei, Q.; Miao, P.; Dong, W.-F. Two-Step Hydrothermal Preparation of Carbon Dots for Calcium Ion Detection. ACS Appl. Mater. Interfaces 2019, 11, 44566–44572. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ge, L.; Li, Y.; Mukhtar, M.; Shen, B.; Yang, D.; Li, J. Carbon dots derived from flax straw for highly sensitive and selective detections of cobalt, chromium, and ascorbic acid. J. Colloid Interface Sci. 2020, 579, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848–126868. [Google Scholar] [CrossRef]
- Lin, M.; Zou, H.Y.; Yang, T.; Liu, Z.X.; Liu, H.; Huang, C.Z. An inner filter effect based sensor of tetracycline hydrochloride as developed by loading photoluminescent carbon nanodots in the electrospun nanofibers. Nanoscale 2016, 8, 2999–3007. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.; Tian, Z.; Lin, Y.; Shi, Z. Facilely synthesized N-doped carbon quantum dots with high fluorescent yield for sensing Fe3+. New J. Chem. 2016, 40, 2083–2088. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, C.; Song, J.; Li, H.; Du, D.; Lin, Y. Drug-Derived Bright and Color-Tunable N-Doped Carbon Dots for Cell Imaging and Sensitive Detection of Fe3+ in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 7399–7405. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Zhang, Q.; Cao, J.; Qian, C.; Ye, J.; Xu, S.; Zhang, Y.; Li, Y. Facile and Green Synthesis of Highly Fluorescent Carbon Quantum Dots from Water Hyacinth for the Detection of Ferric Iron and Cellular Imaging. Nanomaterials 2022, 12, 1528. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, M.; Guo, J.; Song, J.; Zeng, P.; Qu, J.; Chen, Y.; Li, H. Facile Synthesis of Green Fluorescent Carbon Dots and Their Application to Fe3+ Detection in Aqueous Solutions. Nanomaterials 2022, 12, 1487. [Google Scholar] [CrossRef]
- Gan, L.; Su, Q.; Chen, Z.; Yang, X. Exploration of pH-responsive carbon dots for detecting nitrite and ascorbic acid. Appl. Surf. Sci. 2020, 530, 147269–147276. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhu, X.; Liu, L.; Duan, Z.; Liu, Y.; Zhang, W.; Cui, J.; Rong, Y.; Dong, C. One-Step Synthesis of Nitrogen/Fluorine Co-Doped Carbon Dots for Use in Ferric Ions and Ascorbic Acid Detection. Nanomaterials 2022, 12, 2377. https://doi.org/10.3390/nano12142377
Zhao Y, Zhu X, Liu L, Duan Z, Liu Y, Zhang W, Cui J, Rong Y, Dong C. One-Step Synthesis of Nitrogen/Fluorine Co-Doped Carbon Dots for Use in Ferric Ions and Ascorbic Acid Detection. Nanomaterials. 2022; 12(14):2377. https://doi.org/10.3390/nano12142377
Chicago/Turabian StyleZhao, Yan, Xiaoxuan Zhu, Lu Liu, Zhiqing Duan, Yanping Liu, Weiyuan Zhang, Jingjing Cui, Yafang Rong, and Chen Dong. 2022. "One-Step Synthesis of Nitrogen/Fluorine Co-Doped Carbon Dots for Use in Ferric Ions and Ascorbic Acid Detection" Nanomaterials 12, no. 14: 2377. https://doi.org/10.3390/nano12142377
APA StyleZhao, Y., Zhu, X., Liu, L., Duan, Z., Liu, Y., Zhang, W., Cui, J., Rong, Y., & Dong, C. (2022). One-Step Synthesis of Nitrogen/Fluorine Co-Doped Carbon Dots for Use in Ferric Ions and Ascorbic Acid Detection. Nanomaterials, 12(14), 2377. https://doi.org/10.3390/nano12142377