Synthesis and Electrochemical Performance of the Orthorhombic V2O5·nH2O Nanorods as Cathodes for Aqueous Zinc Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterizations
2.3. Electrochemical Measurements
2.4. Differential Electrochemical Mass Spectrometry Measurement
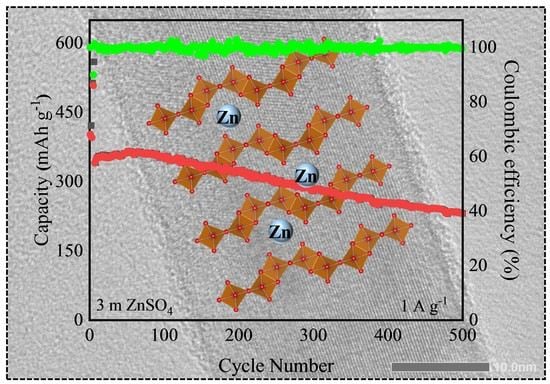
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294. [Google Scholar] [CrossRef] [PubMed]
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Hasa, I.; Passerini, S. Challenges and Strategies for High-Energy Aqueous Electrolyte Rechargeable Batteries. Angew. Chem. Int. Ed. 2021, 60, 598–616. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano-Micro Lett. 2019, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Gao, J.; Xie, X.; Liang, S.; Lu, B.; Zhou, J. Inorganic Colloidal Electrolyte for Highly Robust Zinc-Ion Batteries. Nano-Micro Lett. 2021, 13, 69. [Google Scholar] [CrossRef]
- Daskalakis, S.; Wang, M.; Carmalt, C.J.; Vernardou, D. Electrochemical Investigation of Phenethylammonium Bismuth Iodide as Anode in Aqueous Zn2+ Electrolytes. Nanomaterials 2021, 11, 656. [Google Scholar] [CrossRef]
- Chao, D.; Ye, C.; Xie, F.; Zhou, W.; Zhang, Q.; Gu, Q.; Davey, K.; Gu, L.; Qiao, S.-Z. Atomic Engineering Catalyzed MnO2 Electrolysis Kinetics for a Hybrid Aqueous Battery with High Power and Energy Density. Adv. Mater. 2020, 32, 2001894. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.; Zhong, Y.; Li, Y.; Sun, C.; Han, X.; Deng, Y.; Zhao, N.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Meng, R.; Gao, L.; Zou, Y.; Peng, P.; Shao, Y.; Liang, X. Insights into the Structure Stability of Prussian Blue for Aqueous Zinc Ion Batteries. Energy Environ. Mater. 2021, 4, 111–116. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Alfaruqi, M.H.; Putro, D.Y.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-K.; Kim, J. Na2V6O16·3H2O Barnesite Nanorod: An Open Door to Display a Stable and High Energy for Aqueous Rechargeable Zn-Ion Batteries as Cathodes. Nano Lett. 2018, 18, 2402–2410. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Geiger, D.; Han, J.; Varzi, A.; Kaiser, U.; Moretti, A.; Passerini, S. Calcium vanadate sub-microfibers as highly reversible host cathode material for aqueous zinc-ion batteries. Chem. Commun. 2019, 55, 2265–2268. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975. [Google Scholar] [CrossRef]
- Zhang, S.; Long, S.; Li, H.; Xu, Q. A high-capacity organic cathode based on active N atoms for aqueous zinc-ion batteries. Chem. Eng. J. 2020, 400, 125898. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable Aqueous Zn–V2O5 Battery with High Energy Density and Long Cycle Life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Guo, X.; Fang, G.; Liang, S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef] [PubMed]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, H.; Gao, H.; Liu, Q.; Hu, Z.; Han, L.; Wang, S.; Wu, S.; Fang, S.; Chou, S. In Situ Lattice Tunnel Distortion of Vanadium Trioxide for Enhancing Zinc Ion Storage. Adv. Energy Mater. 2021, 11, 2100973. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Liu, W.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. Operando constructing vanadium tetrasulfide-based heterostructures enabled by extrinsic adsorbed oxygen for enhanced zinc ion storage. J. Mater. Chem. A 2021, 9, 11433–11441. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as Cathode Material for High-Performance Aqueous Zinc Ion Batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-ion Storage Using Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Mengyu, Y.; Pan, H.; Ying, C.; Shanyu, W.; Qiulong, W.; Kangning, Z.; Xu, X.; Qinyou, A.; Yi, S.; Yuyan, S.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, Y.; Wei, Q.; Tan, S.; Xu, Y.; Zhang, G.; Liao, X.; Yang, W.; Li, Q.; An, Q.; et al. Strongly Coupled Pyridine-V2O5·nH2O Nanowires with Intercalation Pseudocapacitance and Stabilized Layer for High Energy Sodium Ion Capacitors. Small 2019, 15, 1900379. [Google Scholar] [CrossRef]
- Qin, H.; Chen, L.; Wang, L.; Chen, X.; Yang, Z. V2O5 hollow spheres as high rate and long life cathode for aqueous rechargeable zinc ion batteries. Electrochim. Acta 2019, 306, 307–316. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Kalambate, P.K.; Zhong, Y.; Huang, Z.; Xie, M.; Shen, Y.; Huang, Y. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Fang, G.; Wang, Z.; Cai, Y.; Tang, B.; Zhou, J.; Liang, S. V2O5 Nanospheres with Mixed Vanadium Valences as High Electrochemically Active Aqueous Zinc-Ion Battery Cathode. Nano-Micro Lett. 2019, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhu, T.; van den Bergh, W.; Stefik, M.; Huang, K. A High Performing Zn-Ion Battery Cathode Enabled by In Situ Transformation of V2O5 Atomic Layers. Angew. Chem. Int. Ed. 2020, 59, 17004–17011. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Zhang, P.; Wang, H.; Li, S.; Ji, S.; Wen, Z.; Sun, J. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl. Surf. Sci. 2020, 502, 144207. [Google Scholar] [CrossRef]
- Cao, A.-M.; Hu, J.-S.; Liang, H.-P.; Wan, L.-J. Self-Assembled Vanadium Pentoxide (V2O5) Hollow Microspheres from Nanorods and Their Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2005, 44, 4391–4395. [Google Scholar] [CrossRef]
- Sun, W.; Gao, G.; Zhang, K.; Liu, Y.; Wu, G. Self-assembled 3D N-CNFs/V2O5 aerogels with core/shell nanostructures through vacancies control and seeds growth as an outstanding supercapacitor electrode material. Carbon 2018, 132, 667–677. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Liu, C.; Song, H.; Nan, X.; Cao, G. The effect of nitrogen annealing on lithium ion intercalation in nickel-doped lithium trivanadate. Sci. Bull. 2016, 61, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Zanarini, S.; Di Lupo, F.; Bedini, A.; Vankova, S.; Garino, N.; Francia, C.; Bodoardo, S. Three-colored electrochromic lithiated vanadium oxides: The role of surface superoxides in the electro-generation of the red state. J. Mater. Chem. C 2014, 2, 8854–8857. [Google Scholar] [CrossRef]
- Zong, Q.; Du, W.; Liu, C.; Yang, H.; Zhang, Q.; Zhou, Z.; Atif, M.; Alsalhi, M.; Cao, G. Enhanced Reversible Zinc Ion Intercalation in Deficient Ammonium Vanadate for High-Performance Aqueous Zinc-Ion Battery. Nano-Micro Lett. 2021, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ren, J.; Yang, X.; Chang, G.; Bu, Y.; Wei, G.; Han, W.; Yang, D. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 9952–9959. [Google Scholar] [CrossRef]
- Li, X.; Kou, Z.; Xi, S.; Zang, W.; Yang, T.; Zhang, L.; Wang, J. Porous NiCo2S4/FeOOH nanowire arrays with rich sulfide/hydroxide interfaces enable high OER activity. Nano Energy 2020, 78, 105230. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Bai, Z.; Lin, L.; Chen, G.; Song, X.; Xiong, D.; Li, D.; Sun, X. Superior sodium storage of novel VO2 nano-microspheres encapsulated into crumpled reduced graphene oxide. J. Mater. Chem. A 2017, 5, 4850–4860. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Qin, B.; Passerini, S. High-Voltage Operation of a V2O5 Cathode in a Concentrated Gel Polymer Electrolyte for High-Energy Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2020, 12, 15305–15312. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. Understanding the Dissolution and Phase Transformation Mechanisms in Aqueous Zn/α-V2O5 Batteries. Chem. Mater. 2021, 33, 4089–4098. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Han, S.-D.; Kim, S.; Lipson, A.L.; Tepavcevic, S.; Fister, T.T.; Bloom, I.D.; Burrell, A.K.; Johnson, C.S. A High Power Rechargeable Nonaqueous Multivalent Zn/V2O5 Battery. Adv. Energy Mater. 2016, 6, 1600826. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, M.; Wang, Y.; Xu, J.; Liu, Y.; Jiao, L.; Zhang, N. Long-Life Zinc/Vanadium Pentoxide Battery Enabled by a Concentrated Aqueous ZnSO4 Electrolyte with Proton and Zinc Ion Co-Intercalation. ACS Appl. Energy Mater. 2020, 3, 11183–11192. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zheng, D.; Ruan, P.; Cai, Y.; Dai, X.; Niu, X.; Pei, C.; Shi, W.; Liu, W.; et al. Ultra-Fast and Scalable Saline Immersion Strategy Enabling Uniform Zn Nucleation and Deposition for High-Performance Zn-Ion Batteries. Small 2021, 17, 2101901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yang, W.; Chen, B.; Zhao, Z.; Qiu, H.; Dong, S.; Zhou, X.; Cui, G.; Chen, L. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Mo, F.; Wang, D.; Liang, G.; Zhao, Y.; Yang, Q.; Huang, Z.; Zhi, C. Calendar Life of Zn Batteries Based on Zn Anode with Zn Powder/Current Collector Structure. Adv. Energy Mater. 2021, 11, 2003931. [Google Scholar] [CrossRef]
- Du, W.; Huang, S.; Zhang, Y.; Ye, M.; Li, C.C. Enable commercial Zinc powders for dendrite-free Zinc anode with improved utilization rate by pristine graphene hybridization. Energy Storage Mater. 2022, 45, 465–473. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, S.; Tao, P.; Shen, T.; Huang, Z.; Yan, J.; Chen, Y. Pillaring Effect of K Ion Anchoring for Stable V2O5-Based Zinc-Ion Battery Cathodes. ChemNanoMat 2020, 6, 797–805. [Google Scholar] [CrossRef]
- Chen, H.; Qin, H.; Chen, L.; Wu, J.; Yang, Z. V2O5@CNTs as cathode of aqueous zinc ion battery with high rate and high stability. J. Alloys Compd. 2020, 842, 155912. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Guo, G.; Wang, K.; Zhang, H. Synthesis and Electrochemical Performance of the Orthorhombic V2O5·nH2O Nanorods as Cathodes for Aqueous Zinc Batteries. Nanomaterials 2022, 12, 2530. https://doi.org/10.3390/nano12152530
Tan X, Guo G, Wang K, Zhang H. Synthesis and Electrochemical Performance of the Orthorhombic V2O5·nH2O Nanorods as Cathodes for Aqueous Zinc Batteries. Nanomaterials. 2022; 12(15):2530. https://doi.org/10.3390/nano12152530
Chicago/Turabian StyleTan, Xiaoping, Gaoli Guo, Kaidi Wang, and Huang Zhang. 2022. "Synthesis and Electrochemical Performance of the Orthorhombic V2O5·nH2O Nanorods as Cathodes for Aqueous Zinc Batteries" Nanomaterials 12, no. 15: 2530. https://doi.org/10.3390/nano12152530
APA StyleTan, X., Guo, G., Wang, K., & Zhang, H. (2022). Synthesis and Electrochemical Performance of the Orthorhombic V2O5·nH2O Nanorods as Cathodes for Aqueous Zinc Batteries. Nanomaterials, 12(15), 2530. https://doi.org/10.3390/nano12152530