CO Methanation over NiO-CeO2 Mixed-Oxide Catalysts Prepared by a Modified Co-Precipitation Method: Effect of the Preparation pH on the Catalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Catalysts Characterization
3. Results
3.1. Catalysts Characterization
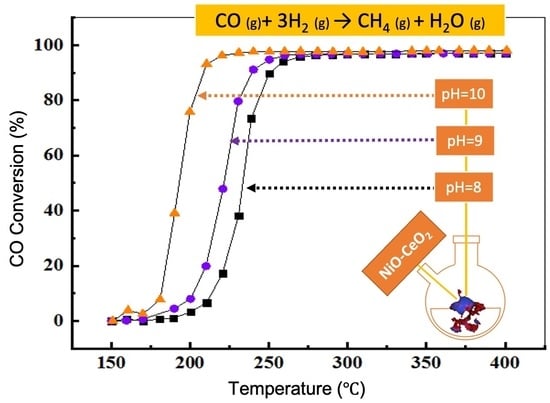
3.2. Catalytic Activity of NiOCeO2-pH in CO Methanation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sehested, J.; Dahl, S.; Jacobsen, J.; Rostrup-Nielsen, J.R. Methanation of CO over nickel: Mechanism and kinetics at high H2/CO ratios. J. Phys. Chem. B 2005, 109, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Rostrup-Nielsen, J.R.; Aasberg-Petersen, K. Fuel Cell Handbook; Wiley: New York, NY, USA, 2003; Volume 3. [Google Scholar]
- Fan, J.; Hong, H.; Jin, H. Biomass and coal co-feed power and SNG polygeneration with chemical looping combustion to reduce carbon footprint for sustainable energy development: Process simulation and thermodynamic assessment. Renew. Energy 2018, 125, 260–269. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Da Silva, D.C.D.; Letichevsky, S.; Borges, L.E.P.; Appel, L.G. The Ni/ZrO2 catalyst and the methanation of CO and CO2. Int. J. Hydrogen Energy 2012, 37, 8923–8928. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Li, F.M.; Cheng, H.M.; Fan, L.Y.; Zhao, Y.X. A comparative study on the catalytic properties of high Ni-loading Ni/SiO2 and low Ni-loading Ni-Ce/SiO2 for CO methanation. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2013, 41, 972–977. [Google Scholar] [CrossRef]
- Takenaka, S.; Shimizu, T.; Otsuka, K. Complete removal of carbon monoxide in hydrogen-rich gas stream through methanation over supported metal catalysts. Int. J. Hydrogen Energy 2004, 29, 1065–1073. [Google Scholar] [CrossRef]
- Liu, J.X.; Su, H.Y.; Li, W.X. Structure sensitivity of CO methanation on Co (0 0 0 1), (1 0 1 - 2) and (1 1 2 - 0) surfaces: Density functional theory calculations. Catal. Today 2013, 215, 36–42. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Pedersen, K.; Sehested, J. High temperature methanation. Sintering and structure sensitivity. Appl. Catal. A Gen. 2007, 330, 134–138. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Selective methanation of CO over supported Ru catalysts. Appl. Catal. B Environ. 2009, 88, 470–478. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Preparation of highly active and stable NiO-CeO2 nanocatalysts for CO selective methanation. Int. J. Hydrogen Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Rombi, E.; Cutrufello, M.G.; Atzori, L.; Monaci, R.; Ardu, A.; Gazzoli, D.; Deiana, P.; Ferino, I. CO methanation on Ni-Ce mixed oxides prepared by hard template method. Appl. Catal. A Gen. 2016, 515, 144–153. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Zhao, B.; Wang, Z.; Wang, Y.; Liu, C.J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Int. J. Hydrogen Energy 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, J.; Han, Y.; Wang, X.; Pei, Y.; Zhang, Z.; Liu, Y.; Ren, J. Highly Active and Dispersed Ni/Al2O3 Catalysts for CO Methanation Prepared by the Cation-Anion Double-Hydrolysis Method: Effects of Zr, Fe, and Ce Promoters. Ind. Eng. Chem. Res. 2019, 58, 11728–11738. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R.; Zhao, Y. Effect of ZrO2 promoter on structure and catalytic activity of the Ni/SiO2 catalyst for CO methanation in hydrogen-rich gases. Catal. Today 2010, 158, 470–474. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, J.; Quan, Y.; Yin, S.; Wu, S.; Ren, J. Highly efficient LaxCe1−xO2−x/2 nanorod-supported nickel catalysts for CO methanation: Effect of la addition. Energy Fuels 2021, 35, 3307–3314. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, Y.; Li, L.; Huang, X.; Li, G. Heterosturcture NiO/Ce1−xNixO2: Synthesis and synergistic effect of simultaneous surface modification and internal doping for superior catalytic performance. RSC Adv. 2014, 4, 6397–6406. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, A.; Kumar, S.; Thakur, A.; Thakur, R.; Bhatia, S.K.; Sharma, A.K. Multifaceted potential applicability of hydrotalcite-type anionic clays from green chemistry to environmental sustainability. Chemosphere 2022, 306, 135464. [Google Scholar] [CrossRef]
- Aberkane, A.B.; Yeste, M.P.; Fayçal, D.; Goma, D.; Cauqui, M.Á. Catalytic soot oxidation activity of NiO-CeO2 catalysts prepared by a coprecipitation method: Influence of the preparation pH on the catalytic performance. Materials 2019, 12, 3436. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.J.M.; Silva, V.D.; Sousa, A.R.O.; Grilo, J.P.F.; Simões, T.A.; Macedo, D.A.; Nascimento, R.M.; Paskocimas, C.A. Battery-like behavior of Ni-ceria based systems: Synthesis, surface defects and electrochemical assessment. Ceram. Int. 2018, 45, 7157–7165. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Nat. Publ. Gr. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, Y.J.; Koo, B.H.; Lee, C.G. Structural and magnetic properties of Ni doped CeO2 nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 7204–7207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yu, F.; Li, J.; Chen, K.; Yao, Y.; Li, P.; Zhu, M.; Shi, Y.; Wang, Q.; Guo, X. High CO methanation performance of two-dimensional Ni/MgAl layered double oxide with enhanced oxygen vacancies via flash nanoprecipitation. Catalysts 2018, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Wang, X. Catalytic performances of NiO-CeO2 for the reforming of methane with CO2 and O2. Fuel 2006, 85, 2243–2247. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Akin, A.N.; Önsan, Z.I.; Trimm, D.L. Structure/activity relationships in coprecipitated nickel-alumina catalysts using CO2 adsorption and methanation. Appl. Catal. A Gen. 1996, 145, 185–193. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel catalysts supported on barium hexaaluminate for enhanced CO methanation. Ind. Eng. Chem. Res. 2012, 51, 10345–10353. [Google Scholar] [CrossRef]







| Samples | Crystalline Phases | Average Crystallite Size (nm) | Lattice Parameter (Å) | Ni (wt%) | SBET (m2/g) | VP (cm3/g) | DP (nm) |
|---|---|---|---|---|---|---|---|
| CeO2-8 | CeO2-fluorite | 12.6 | 5.410 | -- | -- | -- | -- |
| NiOCeO2-8 | NiO-cubic CeO2-fluorite | 18.7 5.2 | 4.170 5.397 | 28.0 | 73 | 0.47 | 25.6 |
| NiOCeO2-9 | NiO-cubic CeO2-fluorite | 14,3 4,2 | 4.173 5.399 | 28.0 | 80 | 0.60 | 30.3 |
| NiOCeO2-10 | NiO-cubic CeO2-fluorite | 13.0 4.8 | 4.169 5.405 | 32.2 | 91 | 0.44 | 19.6 |
| Sample | Ni Dispersion (%) | Ni Particle Size (nm) | Ni Surface Area (m2/g) |
|---|---|---|---|
| NiOCeO2-8 | 8.1 | 12.5 | 15.1 |
| NiOCeO2-9 | 7.7 | 13.2 | 14.3 |
| NiOCeO2-10 | 2.4 | 41.0 | 4.6 |
| Catalysts | CO Conversion (%) | Conversion Rate (mmolCO·gNi−1·h) | TOF (s−1) × 103 |
|---|---|---|---|
| NiOCeO2-8 | 16.7 | 2.9 | 5.9 |
| NiOCeO2-9 | 48.3 | 8.4 | 17.9 |
| NiOCeO2-10 | 96.7 | 14.8 | 101.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendieb Aberkane, A.; Yeste, M.P.; Djazi, F.; Cauqui, M.Á. CO Methanation over NiO-CeO2 Mixed-Oxide Catalysts Prepared by a Modified Co-Precipitation Method: Effect of the Preparation pH on the Catalytic Performance. Nanomaterials 2022, 12, 2627. https://doi.org/10.3390/nano12152627
Bendieb Aberkane A, Yeste MP, Djazi F, Cauqui MÁ. CO Methanation over NiO-CeO2 Mixed-Oxide Catalysts Prepared by a Modified Co-Precipitation Method: Effect of the Preparation pH on the Catalytic Performance. Nanomaterials. 2022; 12(15):2627. https://doi.org/10.3390/nano12152627
Chicago/Turabian StyleBendieb Aberkane, Amar, María Pilar Yeste, Fayçal Djazi, and Miguel Ángel Cauqui. 2022. "CO Methanation over NiO-CeO2 Mixed-Oxide Catalysts Prepared by a Modified Co-Precipitation Method: Effect of the Preparation pH on the Catalytic Performance" Nanomaterials 12, no. 15: 2627. https://doi.org/10.3390/nano12152627
APA StyleBendieb Aberkane, A., Yeste, M. P., Djazi, F., & Cauqui, M. Á. (2022). CO Methanation over NiO-CeO2 Mixed-Oxide Catalysts Prepared by a Modified Co-Precipitation Method: Effect of the Preparation pH on the Catalytic Performance. Nanomaterials, 12(15), 2627. https://doi.org/10.3390/nano12152627