Mechanism Understanding for Size Regulation of Silver Nanowires Mediated by Halogen Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
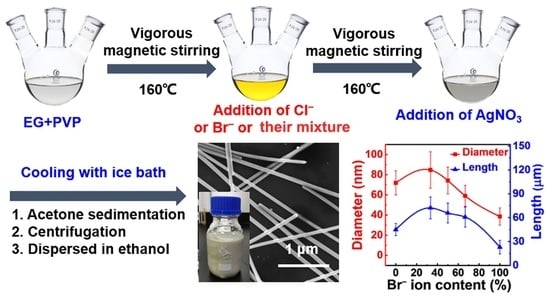
2.2. Synthesis of Silver Nanowires
2.3. Density Function Theory Calculation
2.4. Characterization
3. Results and Discussion
3.1. Growth Characterization of AgNWs in Halogen-Containing Systems
3.2. Analysis of Surface Physicochemical Characteristics of AgNWs
3.3. DFT Calculation for Understanding AgNWs-Halogen Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, S.; Kate, P.R.; Deshpande, J.B.; Kulkarni, A.A. Quantitative understanding of nucleation and growth kinetics of silver nanowires. Chem. Eng. J. 2021, 414, 128711. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, L.; Sowade, E.; Rodriguez, R.D.; Sheremet, E.; Yu, C.M.; Baumann, R.R.; Chen, J.J. Ultra-uniform and very thin Ag nanowires synthesized via the synergy of Cl−, Br− and Fe3+ for transparent conductive films. Nanomaterials 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, B.; Hu, C.; Chen, S.; Liu, X.; Liu, J.; Chen, F.; Chen, J.; Xie, F. A facile method in removal of PVP ligands from silver nanowires for high performance and reusable SERS substrate. Spectrochim. Acta A 2020, 228, 117733. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Ke, S.; Li, Y.; Zhu, X.; Gu, X.; Kan, C.; Shi, D. Silver nanowires deposited on cellulose nanofibers/graphene oxide hybrid membranes as sandwich-structured films for optoelectronic and SERS applications. ACS Appl. Nano Mater. 2020, 3, 10844–10854. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Yang, H.; Zhen, X.; Ma, L.; Williams, D.; Sun, X.; Lang, M.F. Highly transparent and flexible circuits through patterning silver nanowires into microfluidic channels. Chem. Commun. 2018, 54, 4923–4926. [Google Scholar] [CrossRef]
- Subburaj, S.; Arumugam, B.; Chen, S.M.; Chen, T.W.; Seetharam, A.; Ramaraj, S.K. Polyol synthesis of Ag Nanowires as an electrochemical sensor for the quantification of Carcinogenic Hydrazine. Int. J. Electrochem. Sci. 2021, 16, 210773. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Silver nanowires as electron transfer mediators in electrochemical catechol biosensors. Sensors 2021, 21, 899. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, J.; Qin, F.; Yan, Z.; Guo, A.; Kan, C. Highly efficient and stable transparent electromagnetic interference shielding films based on silver nanowires. Nanoscale 2020, 12, 14589–14597. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, A.; Yan, Z.; Qin, F.; Xu, J.; Ji, Y.; Kan, C. PET/Ag NW/PMMA transparent electromagnetic interference shielding films with high stability and flexibility. Nanoscale 2021, 13, 8067–8076. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuat. B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, A.; Xu, J.; Kan, C. The synthesis of silver nanowires with tunable diameters using halide ions for flexible transparent conductive films. CrystEngComm 2020, 22, 8421–8429. [Google Scholar] [CrossRef]
- Ran, Y.; He, W.; Wang, K.; Ji, S.; Ye, C. A one-step route to Ag nanowires with a diameter below 40 nm and an aspect ratio above 1000. Chem. Commun. 2014, 50, 14877–14880. [Google Scholar] [CrossRef]
- Khanarian, G.; Joo, J.; Liu, X.-Q.; Eastman, P.; Werner, D.; O’Connell, K.; Trefonas, P. The optical and electrical properties of silver nanowire mesh films. J. Appl. Phys. 2013, 114, 024302. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating simulations and experiments to predict sheet resistance and optical transmittance in nanowire films for transparent conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef]
- Sorel, S.; Lyons, P.E.; De, S.; Dickerson, J.C.; Coleman, J.N. The dependence of the optoelectrical properties of silver nanowire networks on nanowire length and diameter. Nanotechnology 2012, 23, 185201. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Du, Z.; Zou, W.; Li, H.; Zhang, C. The in situ growth of silver nanowires on multi-walled carbon nanotubes and their application in transparent conductive thin films. RSC Adv. 2014, 4, 27591–27596. [Google Scholar] [CrossRef]
- Sun, X.Y.; Xu, F.Q.; Li, Z.M.; Zhang, W.H. Cyclic voltammetry for the fabrication of high dense silver nanowire arrays with the assistance of AAO template. Mater. Chem. Phys. 2005, 90, 69–72. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Si, X.; He, Z.; Qi, J.; Feng, J.; Cao, J. Single-crystalline silver nanowire arrays directly synthesized onto substrates by template-assisted chemical wetting. Materialia 2020, 9, 100529. [Google Scholar] [CrossRef]
- Zheng, X.J.; Jiang, Z.Y.; Xie, Z.X.; Zhang, S.H.; Mao, B.W.; Zheng, L.S. Growth of silver nanowires by an unconventional electrodeposition without template. Electrochem. Commun. 2007, 9, 629–632. [Google Scholar] [CrossRef]
- Wang, J.; Pan, S. Electrodeposition of vertically standing Ag nanoplates and nanowires on transparent conductive electrode using porous anodic aluminum oxide template. Nanotechnology 2017, 28, 425601. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, C.; Li, J.; Guo, J.; Yang, D.; Wei, J. Green synthesis of silver nanowires via ultraviolet irradiation catalyzed by phosphomolybdic acid and their antibacterial properties. New J. Chem. 2013, 37, 2179–2185. [Google Scholar] [CrossRef]
- Lin, H.Z.; Ohta, T.; Paul, A.; Hutchison, J.A.; Demid, K.; Lebedev, O.; Van Tendeloo, G.; Hofkens, J.; Uji-i, H. Light-assisted nucleation of silver nanowires during polyol synthesis. J. Photochem. Photobiol. A 2011, 221, 220–223. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Liu, J.; Zhang, M.; Qian, Y. Glucose reduction route synthesis of uniform silver nanowires in large-scale. Chem. Lett. 2004, 33, 1160–1161. [Google Scholar] [CrossRef]
- Bari, B.; Lee, J.; Jang, T.; Won, P.; Ko, S.H.; Alamgir, K.; Arshad, M.; Guo, L.J. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 2016, 4, 11365–11371. [Google Scholar] [CrossRef]
- Sharma, D.; Rakshana, D.A.; Balakrishnan, R.M.; JagadeeshBabu, P. One step synthesis of silver nanowires using fructose as a reducing agent and its antibacterial and antioxidant analysis. Mater. Res. Express 2019, 6, 075050. [Google Scholar] [CrossRef]
- Liu, X.L.; Han, S.; Zhang, S.B.; Zhou, S.S.; Jiao, N.; Zhao, H.Y.; Li, J. One-step growth method of silver nanowires in aqueous environment. Mater. Res. Express 2020, 7, 095001. [Google Scholar] [CrossRef]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Li, B.; Ye, S.; Stewart, I.E.; Alvarez, S.; Wiley, B.J. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, J.; Xu, D.; Sun, Y.; Yan, F. One-pot synthesis and purification of ultralong silver nanowires for flexible transparent conductive electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25465–25473. [Google Scholar] [CrossRef]
- Han, M.; Ge, Y.; Liu, J.; Cao, Z.; Li, M.; Duan, X.; Hu, J. Mixed polyvinyl pyrrolidone hydrogel-mediated synthesis of high-quality Ag nanowires for high-performance transparent conductors. J. Mater. Chem. A 2020, 8, 21062–21069. [Google Scholar] [CrossRef]
- Jharimune, S.; Pfukwa, R.; Chen, Z.; Anderson, J.; Klumperman, B.; Rioux, R.M. Chemical identity of poly (N-vinylpyrrolidone) end groups impact shape evolution during the synthesis of Ag nanostructures. J. Am. Chem. Soc. 2020, 143, 184–195. [Google Scholar] [CrossRef]
- Parente, M.; van Helvert, M.; Hamans, R.F.; Verbroekken, R.; Sinha, R.; Bieberle-Hutter, A.; Baldi, A. Simple and fast high-yield synthesis of silver nanowires. Nano Lett. 2020, 20, 5759–5764. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, Y.; Luo, X.; Lin, S.; Hu, J.; Liu, Y. Investigation into the role of poly(vinylpyrrolidone) in the growth of high aspect ratio silver nanowires. Inorg. Chem. Commun. 2021, 128, 108558. [Google Scholar] [CrossRef]
- Fahad, S.; Yu, H.J.; Li, W.; Liu, J.H.; Li, S.B.A.; Fu, J.C.; Ul Amin, B.; Khan, R.U.; Mehmood, S.; Haq, F.; et al. Synthesis of AgNWs using copper bromide as stabilizing agent and oxygen scavenger and their application in conductive thin flms. Mater. Chem. Phys. 2021, 267, 124643. [Google Scholar] [CrossRef]
- Rui, Y.; Zhao, W.; Zhu, D.; Wang, H.; Song, G.; Swihart, M.T.; Wan, N.; Gu, D.; Tang, X.; Yang, Y. Understanding the effects of NaCl, NaBr and their mixtures on silver nanowire nucleation and growth in terms of the distribution of electron traps in silver halide crystals. Nanomaterials 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Lau, K.S.; Chin, S.X.; Tan, S.T.; Lim, F.S.; Chang, W.S.; Yap, C.C.; Jumali, M.H.H.; Zakaria, S.; Chook, S.W.; Chia, C.H. Silver nanowires as flexible transparent electrode: Role of PVP chain length. J. Alloys Compd. 2019, 803, 165–171. [Google Scholar]
- Ge, Y.; Duan, X.; Zhang, M.; Mei, L.; Hu, J.; Hu, W.; Duan, X. Direct room temperature welding and chemical protection of silver nanowire thin films for high performance transparent conductors. J. Am. Chem. Soc. 2018, 140, 193–199. [Google Scholar] [CrossRef]
- Lei, B.W.; Wang, J.; Du, Y.G.; Zhang, K.L. Controlling the size of silver nanowires through one-pot polyol method with trace halide and its effect on kinetic process. Mater. Res. Express 2017, 4, 075052. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanostructures: Control of product morphology with Fe (II) or Fe (III) species. Langmuir 2005, 21, 8077–8080. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tsuji, M.; Jiang, P.; Nishio, M.; Jang, S.M.; Yoon, S.H. Rapid and high-yield synthesis of silver nanowires using air-assisted polyol method with chloride ions. Colloids Surface A 2009, 338, 33–39. [Google Scholar] [CrossRef]
- Zhan, K.; Su, R.; Bai, S.; Yu, Z.; Cheng, N.; Wang, C.; Xu, S.; Liu, W.; Guo, S.; Zhao, X.Z. One-pot stirring-free synthesis of silver nanowires with tunable lengths and diameters via a Fe3+ & Cl− co-mediated polyol method and their application as transparent conductive films. Nanoscale 2016, 8, 18121–18133. [Google Scholar] [PubMed]
- Toybou, D.; Celle, C.; Aude-Garcia, C.; Rabilloud, T.; Simonato, J.-P. A toxicology-informed, safer by design approach for the fabrication of transparent electrodes based on silver nanowires. Environ. Sci. Nano 2019, 6, 684–694. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Mandal, A.; Chakraborty, S.D.; Maiti, A.; Maity, A.; Kuznetsov, D.; Mondal, P.; Senapati, D. Direct experimental observation of salt induced aspect ratio tunable PFPT silver-nanowire formation: SERS-based ppt level Hg2+ sensing from ground water. RSC Adv. 2016, 6, 45279–45289. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J.; Jiang, R. Large-scale synthesis of silver nanowires via a solvothermal method. J. Mater. Sci. Mater. Electron. 2010, 22, 6–13. [Google Scholar] [CrossRef]
- Weng, W.; Wang, S.; Xiao, W.; Lou, X.W. Direct conversion of rice husks to nanostructured SiC/C for CO2 photoreduction. Adv. Mater. 2020, 32, 2001560. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, H.; Weng, W.; Gu, D.; Xiao, W. Interfacial confinement of Ni-V2O3 in molten salts for enhanced electrocatalytic hydrogen evolution. J. Energy Chem. 2020, 50, 280–285. [Google Scholar] [CrossRef]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Prabukumar, C.; Bhat, K.U. Beneficial effect of manganese (II) ions on the morphology of polyol synthesised silver nanowires. Electron. Mater. Lett. 2020, 16, 264–275. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Ou, M.; Huang, Z.; Lin, S.; Tu, Y.; Hu, J. Behind the role of bromide ions in the synthesis of ultrathin silver nanowires. Mater. Lett. 2018, 213, 23–26. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, N.; Chen, Y.; Weng, W.; Chi, X.; Chen, H.; Tang, D.; Zhong, S. Mechanism Understanding for Size Regulation of Silver Nanowires Mediated by Halogen Ions. Nanomaterials 2022, 12, 2681. https://doi.org/10.3390/nano12152681
Xiao N, Chen Y, Weng W, Chi X, Chen H, Tang D, Zhong S. Mechanism Understanding for Size Regulation of Silver Nanowires Mediated by Halogen Ions. Nanomaterials. 2022; 12(15):2681. https://doi.org/10.3390/nano12152681
Chicago/Turabian StyleXiao, Ni, Yinan Chen, Wei Weng, Xiaopeng Chi, Hang Chen, Ding Tang, and Shuiping Zhong. 2022. "Mechanism Understanding for Size Regulation of Silver Nanowires Mediated by Halogen Ions" Nanomaterials 12, no. 15: 2681. https://doi.org/10.3390/nano12152681
APA StyleXiao, N., Chen, Y., Weng, W., Chi, X., Chen, H., Tang, D., & Zhong, S. (2022). Mechanism Understanding for Size Regulation of Silver Nanowires Mediated by Halogen Ions. Nanomaterials, 12(15), 2681. https://doi.org/10.3390/nano12152681