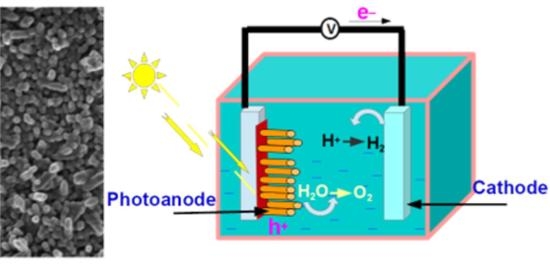
Facile Zn and Ni Co-Doped Hematite Nanorods for Efficient Photocatalytic Water Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Substrate Preparation
2.2. Experimental Procedure
2.3. Characterization
3. Results
3.1. NR Morphology and Seed Layer Thickness
3.2. XRD Structural Characterization
3.3. Raman Characterization
3.4. Elemental Composition
3.5. UV-Vis Absorption
3.6. PEC Measurements
3.6.1. LSV Measurements
3.6.2. Mott–Schottky Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes Based on TiO2 and α-Fe2O3 for Solar Water Splitting-Superior Role of 1D Nanoarchitectures and of Combined Heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1–26. [Google Scholar]
- Nyarige, J.S.; Paradzah, A.T.; Krüger, T.P.J.; Diale, M. Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 366. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards Highly Efficient Photocatalysts Using Semiconductor Nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using Hematite for Photoelectrochemical Water Splitting: A Review of Current Progress and Challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-Electrochemical Hydrogen Generation from Water Using Solar Energy. Materials-Related Aspects. Int. J. Hydrogen Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, C.L.; Lee, W.Y.; Chen, J.; Guo, P.; Zhao, L.; Shen, S. Nb-Doped Hematite Nanorods for Efficient Solar Water Splitting: Electronic Structure Evolution versus Morphology Alteration. ChemNanoMat 2016, 2, 704–711. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.S. Photoelectrochemical Water Splitting with P-Type Metal Oxide Semiconductor Photocathodes. ChemSusChem 2019, 12, 1835–1845. [Google Scholar] [CrossRef]
- Tofanello, A.; Shen, S.; De Souza, F.L.; Vayssieres, L. Strategies to Improve the Photoelectrochemical Performance of Hematite Nanorod-Based Photoanodes. APL Mater. 2020, 8, 040905. [Google Scholar] [CrossRef]
- Singh, A.P.; Saini, N.; Mehta, B.R.; Carraro, G.; Barreca, D. Hematite Thin Film Photoanodes for Visible Light Water Photooxidation: Effects of Zn Doping and Hydrogen Treatment. J. Nanosci. Nanotechnol. 2017, 17, 8959–8966. [Google Scholar] [CrossRef]
- Barroso, M.; Pendlebury, S.R.; Cowan, A.J.; Durrant, J.R. Charge Carrier Trapping, Recombination and Transfer in Hematite (α-Fe2O3) Water Splitting Photoanodes. Chem. Sci. 2013, 4, 2724–2734. [Google Scholar] [CrossRef]
- Kyesmen, P.I.; Nombona, N.; Diale, M. Heterojunction of Nanostructured α-Fe2O3/CuO for Enhancement of Photoelectrochemical Water Splitting. J. Alloys Compd. 2021, 863, 158724. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhu, S.; Liu, Y.; Zhang, X.; Wang, J.-J.; Braun, A.B. Covalent S—O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water SplittingUntEpAlq3mcemibb0x8Q. Small 2021, 17, 30. [Google Scholar]
- Lin, Y.; Yuan, G.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D. Semiconductor Nanostructure-Based Photoelectrochemical Water Splitting: A Brief Review. Chem. Phys. Lett. 2011, 507, 209–215. [Google Scholar] [CrossRef]
- Talibawo, J.; Nyarige, J.S.; Kyesmen, P.I.; Cyulinyana, M.C.; Diale, M. The Behavior of Hydrothermally Synthesized Hematite Nanorods Prepared on Spin Coated Seed Layers. Mater. Res. Express 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Suman; Chahal, S.; Singh, S.; Goel, P.; Kumar, A.; Singh, O.; Kumar, P. Understanding the Role of Ni Ions on the Photocatalytic Activity and Dielectric Properties of Hematite Nanostructures: An Experimental and DFT Approach. J. Phys. Chem. Solids 2021, 156, 110118. [Google Scholar] [CrossRef]
- Rauf, A.; Adil, M.; Mian, S.A.; Rahman, G.; Ahmed, E.; MohyUd Din, Z.; Qun, W. Tuning the Optoelectronic Properties of Hematite with Rhodium Doping for Photoelectrochemical Water Splitting Using Density Function Theory Approach. Sci. Rep. 2021, 11, 41. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.H.; Chen, H.; Lyu, M.; Butburee, T.; Wang, L. Stable Hematite Nanosheet Photoanodes for Enhanced Photoelectrochemical Water Splitting. Adv. Mater. 2016, 28, 6405–6410. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Structural and Optical Properties of Hematite and L-Arginine/Hematite Nanostructures Prepared by Thermal Spray Pyrolysis. Surf. Interfaces 2020, 18, 100394. [Google Scholar] [CrossRef]
- Cui, X.; Liu, T.; Zhang, Z.; Wang, L.; Zuo, S.; Zhu, W. Hematite Nanorods with Tunable Porous Structure: Facile Hydrothermal-Calcination Route Synthesis, Optical and Photocatalytic Properties. Powder Technol. 2014, 266, 113–119. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Ravi, G.; Babu, E.S.; Velauthapillai, D.; Alharbi, S.A. Morphological Exploration of Chemical Vapor–Deposited P-Doped ZnO Nanorods for Efficient Photoelectrochemical Water Splitting. Ceram. Int. 2021, 47, 6521–6527. [Google Scholar] [CrossRef]
- Mulmudi, H.K.; Mathews, N.; Dou, X.C.; Xi, L.F.; Pramana, S.S.; Lam, Y.M.; Mhaisalkar, S.G. Controlled Growth of Hematite (α-Fe2O3) Nanorod Array on Fluorine Doped Tin Oxide: Synthesis and Photoelectrochemical Properties. Electrochem. Commun. 2011, 13, 951–954. [Google Scholar] [CrossRef]
- Ito, N.M.; Carvalho, W.M.; Muche, D.N.F.; Castro, R.H.R.; Dalpian, G.M.; Souza, F.L. High Temperature Activation of Hematite Nanorods for Sunlight Driven Water Oxidation Reaction. Phys. Chem. Chem. Phys. 2017, 19, 25025–25032. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.D.; Li, Y.L.; Wang, T.; Li, Y.; Lu, X.Q.; Gu, J.; Zhao, Y.X.; Song, Y.M.; Tao, C.L. Sn-Doped Hematite Films as Photoanodes for Efficient Photoelectrochemical Water Oxidation. J. Mater. Chem. A 2015, 3, 6751–6755. [Google Scholar] [CrossRef]
- Malviya, K.D.; Klotz, D.; Dotan, H.; Shlenkevich, D.; Tsyganok, A.; Mor, H.; Rothschild, A. Influence of Ti Doping Levels on the Photoelectrochemical Properties of Thin-Film Hematite (α-Fe2O3) Photoanodes. J. Phys. Chem. C 2017, 121, 4206–4213. [Google Scholar] [CrossRef]
- Kumari, S.; Tripathi, C.; Singh, A.P.; Chauhan, D.; Shrivastav, R.; Dass, S.; Satsangi, V.R. Characterization of Zn-Doped Hematite Thin Films for Photoelectrochemical Splitting of Water. Curr. Sci. 2006, 91, 1062–1064. [Google Scholar]
- Li, S.; Cai, J.; Liu, Y.; Gao, M.; Cao, F.; Qin, G. Tuning Orientation of Doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation. Sol. Energy Mater. Sol. Cells 2018, 179, 328–333. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, J.; Huang, H.; Huang, Q.; Zhao, Y.; Li, Y. Enhanced Efficiency of Hematite Photoanode for Water Splitting with the Doping of Ge. Int. J. Hydrogen Energy 2018, 43, 12646–12652. [Google Scholar] [CrossRef]
- Milan, R.; Cattarin, S.; Comisso, N.; Baratto, C.; Kaunisto, K.; Tkachenko, N.V.; Concina, I. Compact Hematite Buffer Layer as a Promoter of Nanorod Photoanode Performances. Sci. Rep. 2016, 6, 35049. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhu, Y.K.; Lv, C.X.; Lai, S.J.; Xu, W.J.; Sun, J.; Sun, Y.Y.; Yang, D.J. Enhanced Visible-Light Photoelectrochemical Performance via Chemical Vapor Deposition of Fe2O3 on a WO3 Film to Form a Heterojunction. Rare Met. 2020, 39, 841–849. [Google Scholar] [CrossRef]
- Bidier, S.A.; Hashim, M.R.; Al-Diabat, A.M.; Bououdina, M. Effect of Growth Time on Ti-Doped ZnO Nanorods Prepared by Low-Temperature Chemical Bath Deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Soltanmoradi, A.; Bakhtiargonbadi, F.; Esfahani, H.; Moakhar, R.S. Effects of Microstructure and Stoichiometry of ZnO Electrospun Seed Layer on the Photoelectrical and Photoelectrochemical Properties of ZnO Nanorods Grown on the FTO Substrate. Mater. Chem. Phys. 2021, 267, 124718. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, S.; Xiao, C.; Li, L.; Li, X. Underlayer Engineering into the Sn-Doped Hematite Photoanode for Facilitating Carrier Extraction. Phys. Chem. Chem. Phys. 2020, 22, 7306–7313. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, Y. Review of Sn-Doped Hematite Nanostructures for Photoelectrochemical Water Splitting. Part. Part. Syst. Charact. 2014, 31, 1113–1121. [Google Scholar] [CrossRef]
- Ahn, H.J.; Yoon, K.Y.; Kwak, M.J.; Park, J.; Jang, J.H. Boron Doping of Metal-Doped Hematite for Reduced Surface Recombination in Water Splitting. ACS Catal. 2018, 8, 11932–11939. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Ling, Y.; Qiu, W.; Wang, F.; Liu, T.; Song, Y.; Liu, X.; Fang, P.; Tong, Y.; et al. Morphology and Doping Engineering of Sn-Doped Hematite Nanowire Photoanodes. Nano Lett. 2017, 17, 2490–2495. [Google Scholar] [CrossRef]
- Xia, C.; Jia, Y.; Tao, M.; Zhang, Q. Tuning the Band Gap of Hematite α-Fe2O3 by Sulfur Doping. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2013, 377, 1943–1947. [Google Scholar] [CrossRef]
- Su, J.; Wang, J.; Liu, C.; Feng, B.; Chen, Y.; Guo, L. On the Role of Metal Atom Doping in Hematite for Improved Photoelectrochemical Properties: A Comparison Study. RSC Adv. 2016, 6, 101745–101751. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Effects of in Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays. Appl. Sci. 2020, 10, 3567. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Meng, L.; Tian, W.; Li, L. Recent Progress on Semiconductor Heterojunction-Based Photoanodes for Photoelectrochemical Water Splitting. Small Sci. 2022, 2, 2100112. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kuo, C.L.; Hsu, Y.K. Facile Preparation of Zn-Doped Hematite Thin Film as Photocathode for Solar Hydrogen Generation. J. Alloys Compd. 2018, 768, 810–816. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Zhao, Z.; Zhao, L.; Claverie, J.P.; Zhang, X.; Wang, J.; Liu, H.; Sang, Y. Efficient Photo-Electrochemical Water Splitting Based on Hematite Nanorods Doped with Phosphorus. Appl. Catal. B Environ. 2019, 248, 388–393. [Google Scholar] [CrossRef]
- Krehula, S.; Ristić, M.; Mitar, I.; Wu, C.; Li, X.; Jiang, L.; Wang, J.; Sun, G.; Zhang, T.; Perović, M.; et al. Synthesis and Properties of Ni-Doped Goethite and Ni-Doped Hematite Nanorods. Croat. Chem. Acta 2018, 91, 389–401. [Google Scholar]
- Li, Q.; Bian, J.; Zhang, N.; Ng, D.H.L. Loading Ni(OH)2 on the Ti-Doped Hematite Photoanode for Photoelectrochemical Water Splitting. Electrochim. Acta 2015, 155, 383–390. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, Y.; Toth, R.; Fortunato, G.; Braun, A. A Facile Nonpolar Organic Solution Process of a Nanostructured Hematite Photoanode with High Efficiency and Stability for Water Splitting. J. Mater. Chem. A 2016, 4, 2821–2825. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, K.; Shin, K.; Moon, J.H.; Lee, T.W.; Park, J.H. Constructing Inverse Opal Structured Hematite Photoanodes via Electrochemical Process and Their Application to Photoelectrochemical Water Splitting. Phys. Chem. Chem. Phys. 2013, 15, 11717–11722. [Google Scholar] [CrossRef] [PubMed]
- Pourghahramani, P.; Forssberg, E. Microstructure Characterization of Mechanically Activated Hematite Using XRD Line Broadening. Int. J. Miner. Process. 2006, 79, 106–119. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.H.; Choi, W.H.; Moon, B.C.; Kong, H.J.; Kang, J.K. Cobalt-Phosphate Catalysts with Reduced Bivalent Co-Ion States and Doped Nitrogen Atoms Playing as Active Sites for Facile Adsorption, Fast Charge Transfer, and Robust Stability in Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 44366–44374. [Google Scholar] [CrossRef] [PubMed]
- Kyesmen, P.I.; Nombona, N.; Diale, M. Modified Annealing Approach for Preparing Multi-Layered Hematite Thin Films for Photoelectrochemical Water Splitting. Mater. Res. Bull. 2020, 131, 110964. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; García-Granda, S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis and Characterization of Ni-Doped α-Fe2O3 Nanoparticles through Co-Precipitation Method with Enhanced Photocatalytic Activities. J. Mater. Sci. Mater. Electron. 2018, 29, 5726–5737. [Google Scholar] [CrossRef]
- Amer, M.A.; Meaz, T.M.; Attalah, S.S.; Ghoneim, A.I. Annealing Effect on Structural Phase Transition of As-Synthesized Mg0.1Sr0.1Mn0.8Fe2O4 Nanoparticles. J. Alloys Compd. 2016, 654, 45–55. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Yogi, A.; Varshney, D. Cu Doping Effect of Hematite (α-Fe2−XCuxO3): Effect on the Structural and Magnetic Properties. Mater. Sci. Semicond. Process. 2014, 21, 38–44. [Google Scholar] [CrossRef]
- Yogi, A.; Varshney, D. Magnetic and Structural Properties of Pure and Cr-Doped Haematite: α-Fe2−xCrxO3 (0 ≤ x ≤ 1). J. Adv. Ceram. 2013, 2, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Bellido-aguilar, D.A.; Tofanello, A.; Souza, F.L.; Negri, L.; José, C.; Constantino, L. Effect of Thermal Treatment on Solid–Solid Interface of Hematite Thin Fi Lm Synthesized by Spin-Coating Deposition Solution. Thin Solid Film. 2016, 604, 28–39. [Google Scholar] [CrossRef]
- Group, S.S. Differing Effects of Particle Size and Shape in the Infrared and Raman Spectra of Kaolinite. Clay Miner. 1998, 33, 601–604. [Google Scholar]
- Tamirat, A.G.; Dubale, A.A.; Su, W.N.; Chen, H.M.; Hwang, B.J. Sequentially Surface Modified Hematite Enables Lower Applied Bias Photoelectrochemical Water Splitting. Phys. Chem. Chem. Phys. 2017, 19, 20881–20890. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Effects of L-Arginine Concentration on Hematite Nanostructures Synthesized by Spray Pyrolysis and Chemical Bath Deposition. Phys. B Condens. Matter 2020, 581, 411924. [Google Scholar] [CrossRef]
- Formal, F.L.; Grätzel, M.; Sivula, K. Controlling Photoactivity in Ultrathin Hematite Films for Solar Water-Splitting. Adv. Funct. Mater. 2010, 20, 1099–1107. [Google Scholar] [CrossRef]
- Zandi, O.; Hamann, T.W. The Potential versus Current State of Water Splitting with Hematite. Phys. Chem. Chem. Phys. 2015, 17, 22485–22503. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Wang, C.; Mao, X.; Wang, Y.; Yang, Y.; Liu, G. Visible Light Induced Photocatalytic Degradation of Rhodamine B on One-Dimensional Iron Oxide Particles. J. Phys. Chem. C 2010, 114, 17051–17061. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Ibrahim, E.; Chong, M.N.; Zhu, T.; Lee, B.K.; Ocon, J.D.; Chan, E.S. In Situ Ni-Doping during Cathodic Electrodeposition of Hematite for Excellent Photoelectrochemical Performance of Nanostructured Nickel Oxide-Hematite p-n Junction Photoanode. Appl. Surf. Sci. 2017, 392, 144–152. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, B.A.; Pan, C.J.; Su, W.N.; Chen, H.M.; Rick, J.; Hwang, B.J. Facile One-Pot Controlled Synthesis of Sn and C Codoped Single Crystal TiO2 Nanowire Arrays for Highly Efficient Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2015, 163, 478–486. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, J.; Guo, P.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Effect of Cr Doping on the Photoelectrochemical Performance of Hematite Nanorod Photoanodes. Nano Energy 2012, 1, 732–741. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, C.; Li, Y.; Han, W.; Fu, W.; He, Y.; Xie, E. Enhanced Charge Separation and Transfer through Fe2O3/ITO Nanowire Arrays Wrapped with Reduced Graphene Oxide for Water-Splitting. Nano Energy 2016, 30, 892–899. [Google Scholar] [CrossRef]
- Gahlawat, S.; Singh, J.; Yadav, A.K.; Ingole, P.P. Exploring Burstein-Moss Type Effects in Nickel Doped Hematite Dendrite Nanostructures for Enhanced Photo-Electrochemical Water Splitting. Phys. Chem. Chem. Phys. 2019, 21, 20463–20477. [Google Scholar] [CrossRef]
- Mirbagheri, N.; Wang, D.; Peng, C.; Wang, J.; Huang, Q.; Fan, C.; Ferapontova, E.E. Visible Light Driven Photoelectrochemical Water Oxidation by Zn- and Ti-Doped Hematite Nanostructures. ACS Catal. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, A.P.; Tripathi, C.; Chauhan, D.; Dass, S.; Shrivastav, R.; Gupta, V.; Sreenivas, K.; Satsangi, V.R. Enhanced Photoelectrochemical Response of Zn-Dotted Hematite. Int. J. Photoenergy 2007, 2007, 087467. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, C.; Zhang, X. Ti, Zn Co-Doped Hematite Photoanode for Solar Driven Photoelectrochemical Water Oxidation. J. Energy Chem. 2019, 35, 30–36. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.X.; Zhang, W.D. Photoelectrochemical Properties of Ni-Doped Fe 2O 3 Thin Films Prepared by Electrodeposition. Electrochim. Acta 2012, 59, 121–127. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, J.; Dong, C.L.; Hu, Y.; Tseng, E.N.; Guo, P.; Guo, L.; Mao, S.S. Surface Engineered Doping of Hematite Nanorod Arrays for Improved Photoelectrochemical Water Splitting. Sci. Rep. 2014, 4, 6627. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Gracia-Espino, E.; Annamalai, A.; Lee, H.H.; Lee, S.Y.; Choi, S.H.; Jang, J.S. Effect of Tetravalent Dopants on Hematite Nanostructure for Enhanced Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2018, 427, 1203–1212. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jun, H.; Hong, S.J.; Kim, H.G.; Lee, J.S. Charge Transfer in Iron Oxide Photoanode Modified with Carbon Nanotubes for Photoelectrochemical Water Oxidation: An Electrochemical Impedance Study. Int. J. Hydrogen Energy 2011, 36, 9462–9468. [Google Scholar] [CrossRef]











| NR Samples | Element (at%) | ||||
|---|---|---|---|---|---|
| O | Fe | Sn | Zn | Ni | |
| Pristine hematite NRs | 70.90 | 14.00 | 15.10 | - | - |
| 3 mM Zn NRs | 70.75 | 13.26 | 15.94 | 0.05 | - |
| 3 Mm Zn/3 Mm Ni NRs | 69.76 | 17.23 | 12.93 | 0.04 | 0.04 |
| 3 Mm Zn/5 Mm Ni NRs | 70.18 | 13.12 | 16.49 | 0.10 | 0.11 |
| 3 Mm Zn/7 Mm Ni NRs | 69.87 | 14.09 | 15.78 | 0.07 | 0.19 |
| 3 Mm Zn/3 Mm Ni NRs | 70.50 | 12.60 | 16.58 | 0.04 | 0.28 |
| NR Sample | (V) | × 1019 (cm−3) |
|---|---|---|
| Pristine hematite NRs | −0.43 | 1.38 |
| 3 mM Zn NRs | −0.02 | 1.07 |
| 3 Mm Zn/3 Mm Ni NRs | −0.36 | 1.55 |
| 3 mM Zn/5 mM Ni NRs | −0.38 | 2.01 |
| 3 mM Zn/7 mM Ni NRs | −0.65 | 2.89 |
| 3 mM Zn/9 mM Ni NRs | −0.70 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talibawo, J.; Kyesmen, P.I.; Cyulinyana, M.C.; Diale, M. Facile Zn and Ni Co-Doped Hematite Nanorods for Efficient Photocatalytic Water Oxidation. Nanomaterials 2022, 12, 2961. https://doi.org/10.3390/nano12172961
Talibawo J, Kyesmen PI, Cyulinyana MC, Diale M. Facile Zn and Ni Co-Doped Hematite Nanorods for Efficient Photocatalytic Water Oxidation. Nanomaterials. 2022; 12(17):2961. https://doi.org/10.3390/nano12172961
Chicago/Turabian StyleTalibawo, Joan, Pannan I. Kyesmen, Marie C. Cyulinyana, and Mmantsae Diale. 2022. "Facile Zn and Ni Co-Doped Hematite Nanorods for Efficient Photocatalytic Water Oxidation" Nanomaterials 12, no. 17: 2961. https://doi.org/10.3390/nano12172961
APA StyleTalibawo, J., Kyesmen, P. I., Cyulinyana, M. C., & Diale, M. (2022). Facile Zn and Ni Co-Doped Hematite Nanorods for Efficient Photocatalytic Water Oxidation. Nanomaterials, 12(17), 2961. https://doi.org/10.3390/nano12172961