Carbon Dots Embedded Hybrid Microgel with Phenylboronic Acid as Monomer for Fluorescent Glucose Sensing and Glucose-Triggered Insulin Release at Physiological pH
Abstract
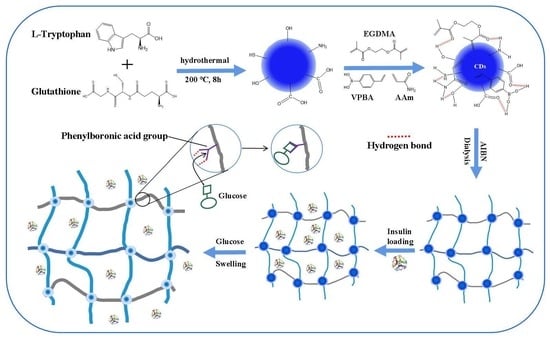
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Synthesis of Poly(VPBA-AAm)-CD Hybrid Microgel
2.3. Fluorescence Deternination Procedure
2.4. Insulin Loading on Poly(VPBA-AAm)-CD Microgels
2.5. Insulin Release In Vitro under Glucose-Controlled Conditions
2.6. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results
3.1. Structure Characterization
3.2. Fluorescence Properties of Poly(VPBA-AAm)-CD
3.3. Fluorescent Glucose Sensing of Poly(VPBA-AAm)-CD Hybrid Microgel
3.4. Glucose-Triggered Insulin Release of Poly(VPBA-AAm)-CD
3.5. Cytotoxicity of Poly(VPBA-AAm)-CD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Effoe, V.S.; McClendon, E.E.; Rodriguez, C.J.; Wagenknecht, L.E.; Evans, G.W.; Chang, P.P.; Bertoni, A.G. Diabetes status modifies the association between carotid intima-media thickness and incident heart failure: The Atherosclerosis Risk in Communities study. Diabetes Res. Clin. Pract. 2017, 128, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.R.; Zinman, B.; Bolli, G.B. Insulins today and beyond. Lancet 2001, 358, 739–746. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Yu, Z. Microneedles for transdermal delivery of insulin. J. Drug Deliv. Sci. Technol. 2015, 28, 11–17. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef]
- Wang, H.; Yi, J.; Yu, Y.; Zhou, S. NIR upconversion fluorescence glucose sensing and glucose-responsive insulin release of carbon dot-immobilized hybrid microgels at physiological pH. Nanoscale 2016, 9, 509–516. [Google Scholar] [CrossRef]
- Cowart, K. A Review of the First Long-term Implantable Continuous Glucose Monitoring System Available in the United States. J. Diabetes Sci. Technol. 2019, 15, 160–166. [Google Scholar] [CrossRef]
- Felix, S.; Grace, A.N.; Jayavel, R. Sensitive electrochemical detection of glucose based on Au-CuO nanocomposites. J. Phys. Chem. Solids 2018, 122, 255–260. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Facklam, A.L.; Cortinas, A.B.; Lu, Y.-C.; Matranga, M.A.; MacIsaac, C.; Hill, M.C.; Langer, R.; Anderson, D.G. Microgel encapsulated nanoparticles for glucose-responsive insulin delivery. Biomaterials 2020, 267, 120458. [Google Scholar] [CrossRef]
- Liu, S.; Chang, C.N.; Verma, M.S.; Hileeto, D.; Müntz, A.; Stahl, U.; Woods, J.; Jones, L.W.; Gu, F.X. Phenylboronic acid modified mucoadhesive nanoparticle drug carriers facilitate weekly treatment of experimentallyinduced dry eye syndrome. Nano Res. 2014, 8, 621–635. [Google Scholar] [CrossRef]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2013, 5, 1503–1518. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, D.; Yao, D.; Guo, R.; Wang, W.; Dong, A.; Kong, D.; Zhang, J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017, 64, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, K.; You, K.; Gao, Z.; Kakuchi, T.; Feng, B.; Duan, Q. Synthesis and characterization of phenylboronic acid-containing polymer for glucose-triggered drug delivery+. Sci. Technol. Adv. Mater. 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holz, E.; Rajagopal, K. In Situ-Forming Glucose-Responsive Hydrogel from Hyaluronic Acid Modified with a Boronic Acid Derivative. Macromol. Chem. Phys. 2020, 221, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.; Losego, M.D.; Braun, P.V. Hydrogel-Based Glucose Sensors: Effects of Phenylboronic Acid Chemical Structure on Response. Chem. Mater. 2013, 25, 3239–3250. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.; Sun, G.; Li, Y.; Guan, X.; Yang, C.; Ngai, T. Engineering hybrid microgels as particulate emulsifiers for reversible Pickering emulsions. Chem. Sci. 2021, 13, 39–43. [Google Scholar] [CrossRef]
- Shin, D.S.; Tokuda, E.Y.; Leight, J.L.; Miksch, C.E.; Brown, T.E.; Anseth, K.S. Synthesis of Microgel Sensors for Spatial and Temporal Monitoring of Protease Activity. ACS Biomater. Sci. Eng. 2017, 4, 378–387. [Google Scholar] [CrossRef]
- Fujii, S.; Armes, S.P.; Binks, B.P.; Murakami, R. Stimulus-Responsive Particulate Emulsifiers Based on Lightly Cross-Linked Poly(4-vinylpyridine)−Silica Nanocomposite Microgels. Langmuir 2006, 22, 6818–6825. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Han, G.; Liu, Z.; Liu, C.; Zhang, C.; Liu, B.; Jiang, C.; Liu, R.; Zhao, T.; et al. Real-Time Discrimination and Versatile Profiling of Spontaneous Reactive Oxygen Species in Living Organisms with a Single Fluorescent Probe. J. Am. Chem. Soc. 2016, 138, 3769–3778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, Y.; Yang, X.; Shi, N.; Geng, L.; Wang, S.; Zhang, J.; Shi, S. High drug-loading system of hollow carbon dots–doxorubicin: Preparation, in vitro release and pH-targeted research. J. Mater. Chem. B 2019, 7, 2130–2137. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon Dots Rooted Agarose Hydrogel Hybrid Platform for Optical Detection and Separation of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, A.; Tian, Y. Functional Surface Engineering of C-Dots for Fluorescent Biosensing and in Vivo Bioimaging. Accounts Chem. Res. 2013, 47, 20–30. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Döring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels—Structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Wu, W.; Chen, S.; Hu, Y.; Zhou, S. A Fluorescent Responsive Hybrid Nanogel for Closed-Loop Control of Glucose. J. Diabetes Sci. Technol. 2012, 6, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Li, Y.; Zhu, H.; Banerjee, P.; Zhou, S. Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 2012, 33, 7115–7125. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, X.; Hu, Y.; Si, Y.; Ding, L.; Wu, W. A fluorescent double-network-structured hybrid nanogel as embeddable nanoglucometer for intracellular glucometry. Biomater. Sci. 2013, 1, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xie, J.; Yan, S.; Jiang, X.; Ye, T.; Wu, W. Graphene@Poly(phenylboronic acid)s Microgels with Selectively Glucose-Responsive Volume Phase Transition Behavior at a Physiological pH. Macromolecules 2014, 47, 6055–6066. [Google Scholar] [CrossRef]
- Wang, H.; Yi, J.; Velado, D.; Yu, Y.; Zhou, S. Immobilization of Carbon Dots in Molecularly Imprinted Microgels for Optical Sensing of Glucose at Physiological pH. ACS Appl. Mater. Interfaces 2015, 7, 15735–15745. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-Modification Integration: One-Step Fabrication of Boronic Acid Functionalized Carbon Dots for Fluorescent Blood Sugar Sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Dang, T.T.; Ma, M.; Tang, B.C.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D.G. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Wu, X.; Zhu, J.; Hu, W.; El Jaouhari, A.; Liu, X. Facile Preparation of Fluorescent Carbon Dots from Glutathione and l-Tryptophan for Sensitive and Selective Off/On Detection of Fe3+ Ions in Serum and Their Bioimaging Application. ACS Omega 2022, 7, 7853–7864. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhang, W.; Wei, S.; Dang, H. The influence of mPEG-PCL and mPEG-PLGA on encapsulation efficiency and drug-loading of SN-38 NPs. Artif. Cells, Nanomedicine, Biotechnol. 2016, 45, 389–397. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Voit, B.; Karak, N. Carbon dot reduced Cu2O nanohybrid/hyperbranched epoxy nanocomposite: Mechanical, thermal and photocatalytic activity. RSC Adv. 2014, 4, 58453–58459. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kobayashi, H.; Kubo, Y. Dansyl-containing boronate hydrogel film as fluorescent chemosensor of copper ions in water. RSC Adv. 2012, 2, 6555–6561. [Google Scholar] [CrossRef]
- Pereira, R.M.; Andrade, G.S.S.; De Castro, H.F.; Campos, M.G.N. Performance of Chitosan/Glycerol Phosphate Hydrogel as a Support for Lipase Immobilization. Mater. Res. 2017, 20, 190–201. [Google Scholar] [CrossRef]
- Lin, H.J.; Herman, P.; Kang, J.S.; Lakowicz, J.R. Fluorescence Lifetime Characterization of Novel Low-pH Probes. Anal. Biochem. 2001, 294, 118–125. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, X.; Shi, A.; Wu, J. Bioresponsive Functional Phenylboronic Acid-Based Delivery System as an Emerging Platform for Diabetic Therapy. Int. J. Nanomed. 2021, 16, 297–314. [Google Scholar] [CrossRef]
- Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.D.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 2018, 172, 234–248.e17. [Google Scholar] [CrossRef]
- Petersen, M.; Madiraju, A.K.; Gassaway, B.; Marcel, M.; Nasiri, A.R.; Butrico, G.; Marcucci, M.J.; Zhang, D.; Abulizi, A.; Zhang, X.-M.; et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016, 126, 4361–4371. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.S.; Goldstein, J.L. Selective versus Total Insulin Resistance: A Pathogenic Paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.F.; Majumdar, S.K.; Kumashiro, N.; Petersen, M.C.; Rahimi, Y.; Gattu, A.K.; Bears, M.; Camporez, J.-P.G.; Cline, G.W.; Jurczak, M.J.; et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA 2015, 112, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Yang, L.Q.; Wang, P.P.; Dong, L.; Hu, X.S.; Chen, F. Research Progress on the Toxicity of Acrylamide. J. Chin. Inst. Food Sci. Technol. 2018, 18, 274–283. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Nie, J.; Ji, Z.; Xu, J.; Zhang, X.; Du, B. Thermosensitive Ionic Microgels via Surfactant-Free Emulsion Copolymerization and in Situ Quaternization Cross-Linking. ACS Appl. Mater. Interfaces 2014, 6, 4498–4513. [Google Scholar] [CrossRef]
- Wang, J.; Hao, H.; Cai, J.H. Amphiphilic Drug Delivery Microcapsules via Layer-by-Layer Self-Assembly. J. Macromol. Sci. Part B 2019, 58, 535–550. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 7, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.K.; Ghosh, M.; Das, P.K. Phenylboronic Acid Appended Pyrene-Based Low-Molecular-Weight Injectable Hydrogel: Glucose-Stimulated Insulin Release. Chem.-A Eur. J. 2015, 21, 12042–12052. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Sánchez-Iglesias, A.; Karg, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Pacifico, J.; Hellweg, T.; Fernández-Barbero, A.; Liz-Marzán, L.M. Encapsulation and Growth of Gold Nanoparticles in Thermoresponsive Microgels. Adv. Mater. 2008, 20, 1666–1670. [Google Scholar] [CrossRef]
- Xue, D.; Xia, H.; Yan, W.; Zhang, J.; Mu, S. Defect Engineering on Carbon-Based Catalysts for Electrocatalytic CO2 Reduction. Nano-Micro Lett. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- Wu, H.; Pang, L.-F.; Fu, M.-J.; Guo, X.-F.; Wang, H. Boron and nitrogen codoped carbon dots as fluorescence sensor for Fe3+ with improved selectivity. J. Pharm. Biomed. Anal. 2019, 180, 113052. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, W.; Zhang, B.; Zhou, D.; Fan, X.; Wang, X.; Liu, X. Carbon Dots Embedded Hybrid Microgel with Phenylboronic Acid as Monomer for Fluorescent Glucose Sensing and Glucose-Triggered Insulin Release at Physiological pH. Nanomaterials 2022, 12, 3065. https://doi.org/10.3390/nano12173065
Zhu J, Liu W, Zhang B, Zhou D, Fan X, Wang X, Liu X. Carbon Dots Embedded Hybrid Microgel with Phenylboronic Acid as Monomer for Fluorescent Glucose Sensing and Glucose-Triggered Insulin Release at Physiological pH. Nanomaterials. 2022; 12(17):3065. https://doi.org/10.3390/nano12173065
Chicago/Turabian StyleZhu, Jinhua, Wei Liu, Bowen Zhang, Danyang Zhou, Xiangze Fan, Xiaoge Wang, and Xiuhua Liu. 2022. "Carbon Dots Embedded Hybrid Microgel with Phenylboronic Acid as Monomer for Fluorescent Glucose Sensing and Glucose-Triggered Insulin Release at Physiological pH" Nanomaterials 12, no. 17: 3065. https://doi.org/10.3390/nano12173065
APA StyleZhu, J., Liu, W., Zhang, B., Zhou, D., Fan, X., Wang, X., & Liu, X. (2022). Carbon Dots Embedded Hybrid Microgel with Phenylboronic Acid as Monomer for Fluorescent Glucose Sensing and Glucose-Triggered Insulin Release at Physiological pH. Nanomaterials, 12(17), 3065. https://doi.org/10.3390/nano12173065