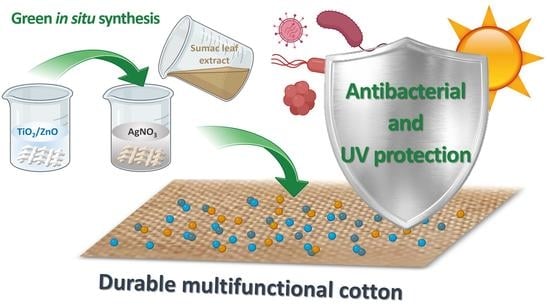
Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CO/TiO2/Ag, CO/ZnO/Ag and CO/TiO2 + ZnO/Ag Samples
2.3. Washing
2.4. Analysis and Measurement
2.4.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.4.2. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.4.3. X-ray Diffraction (XRD)
2.4.4. Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS)
2.4.5. UV–Vis Spectroscopy
2.4.6. Antibacterial Activity
2.4.7. UV Protection Properties
2.4.8. Photocatalytic Activity
3. Results and Discussion
3.1. Morphological, Chemical and Optical Properties
3.2. Functional Properties
3.2.1. Antibacterial Properties
3.2.2. UV Protection Properties
3.2.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckelman, M.J.; Zimmerman, J.B.; Anastas, P.T. Toward green nano: E-factor analysis of several nanomaterial syntheses. J. Ind. Ecol. 2008, 12, 316–328. [Google Scholar] [CrossRef]
- Sarvalkar, P.D.; Barawkar, S.D.; Karvekar, O.S.; Patil, P.D.; Prasad, S.R.; Sharma, K.K.; Prasad, N.R.; Vhatkar, R.S. A review on multifunctional nanotechnological aspects in modern textile. J. Text. Inst. 2022, 1–18. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent advances in TiO2-functionalized textile surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral potential of nanoparticles-can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albo, Y.; Pagliaro, M. New antivirals and antibacterials based on silver nanoparticles. ChemMedChem 2020, 15, 1619–1623. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide−from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, N.S.; Mittal, A.; Sharma, S. TiO2 and its composites as promising biomaterials: A review. Biometals 2018, 31, 147–159. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Functional responses of human adipose tissue-derived mesenchymal stem cells to metal oxide nanoparticles in vitro. J. Biomed. Nanotechnol. 2013, 9, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Milošević, M.; Radoičić, M.; Šaponjić, Z.; Nunney, T.; Deeks, C.; Lazić, V.; Mitrić, M.; Radetić, T.; Radetić, M. In situ photoreduction of Ag+-ions by TiO2 nanoparticles deposited on cotton and cotton/PET fabrics. Cellulose 2014, 21, 3781–3795. [Google Scholar] [CrossRef]
- Gorjanc, M.; Šala, M. Durable antibacterial and UV protective properties of cellulose fabric functionalized with Ag/TiO2 nanocomposite during dyeing with reactive dyes. Cellulose 2016, 23, 2199–2209. [Google Scholar] [CrossRef]
- Mishra, A.; Butola, B.S. Deposition of Ag doped TiO2 on cotton fabric for wash durable UV protective and antibacterial properties at very low silver concentration. Cellulose 2017, 24, 3555–3571. [Google Scholar] [CrossRef]
- Moridi Mahdieh, Z.; Shekarriz, S.; Afshar Taromi, F.; Montazer, M. A new method for in situ synthesis of Ag–TiO2 nanocomposite particles on polyester/cellulose fabric by photoreduction and self-cleaning properties. Cellulose 2018, 25, 2355–2366. [Google Scholar] [CrossRef]
- Chand, K.; Cao, D.; Fouad, D.E.; Shah, A.H.; Lakhan, M.N.; Dayo, A.Q.; Sagar, H.J.; Zhu, K.; Mohamed, A.M.A. Photocatalytic and antimicrobial activity of biosynthesized silver and titanium dioxide nanoparticles: A comparative study. J. Mol. Liq. 2020, 316, 113821. [Google Scholar] [CrossRef]
- Gorguluer, H.; Cakiroglu, B.; Ozacar, M. Ag NPs deposited TiO2 coating material for superhydrophobic, antimicrobial and self-cleaning surface fabrication on fabric. J. Coat. Technol. Res. 2021, 18, 569–579. [Google Scholar] [CrossRef]
- Rashid, M.M.; Tomšič, B.; Simončič, B.; Jerman, I.; Štular, D.; Zorc, M. Sustainable and cost-effective functionalization of textile surfaces with Ag-doped TiO2/polysiloxane hybrid nanocomposite for UV protection, antibacterial and self-cleaning properties. Appl. Surf. Sci. 2022, 595, 153521. [Google Scholar] [CrossRef]
- Avazpour, S.; Karimi, L.; Zohoori, S. Simultaneous coloration and functional finishing of cotton fabric using Ag/ZnO nanocomposite. Color Technol. 2017, 133, 423–430. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Ferreira, A.; Vaz, F.; Fangueiro, R. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials 2018, 8, 1069. [Google Scholar] [CrossRef] [Green Version]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci. Rep. 2020, 10, 5410. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kim, J.; Park, S.; Jeong, Y.W.; Lee, C.; Oh, S.G. Fabrication of Ag-doped ZnO/PAN composite nanofibers by electrospinning: Photocatalytic and antiviral activities. Korean J. Chem. Eng. 2022, 39, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yu, S.F.; Lau, S.P.; Zhang, X.W.; Sun, D.D.; Jun, G. Direct Growth of ZnO Nanocrystals onto the Surface of Porous TiO2 Nanotube Arrays for Highly Efficient and Recyclable Photocatalysts. Small 2009, 5, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.L.; Song, H.S.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Liu, Q.Y.; Shu, S.X.; Xie, Y.; Zhao, Y.Q.; Chen, B.Y.; Dong, W.J. Preparation and characterization of regenerated cellulose/TiO2/ZnO nanocomposites and its photocatalytic activity. Mater. Lett. 2014, 117, 234–236. [Google Scholar] [CrossRef]
- Pan, L.; Shen, G.Q.; Zhang, J.W.; Wei, X.C.; Wang, L.; Zou, J.J.; Zhang, X.W. TiO2−ZnO Composite Sphere Decorated with ZnO Clusters for Effective Charge Isolation in Photocatalysis. Ind. Eng. Chem. Res. 2015, 54, 7226–7232. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Wang, Y.F.; Kang, Y.H.; Wang, C.; Yang, S.B. Fabrication of TiO2/ZnO composite nanofibers with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 7834–7838. [Google Scholar] [CrossRef]
- Siwinska-Stefanska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Gutarowska, B.; Szulc, J.; Matyjas-Zgondek, E.; Kulpinski, P.; Pielech-Przybylska, K.; Rygala, A.; Jachowicz, A.; Rutkowski, E. Cotton Terry Textiles with Photo- and Bio-Activity in a Model Study and Real Conditions. Materials 2020, 13, 3334. [Google Scholar] [CrossRef]
- Rilda, Y.; Damara, D.; Putri, Y.E.; Refinel, R.; Agustein, A.; Pardi, H. Pseudomonas aeruginosa antibacterial textile cotton fiber construction based on ZnO-TiO2 nanorods template. Heliyon 2020, 6, e03710. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Shukla, S.R.; Prasad, V. ZnO-TiO2 hybrid nanocrystal-loaded, wash durable, multifunction cotton textiles. Cellulose 2022, 29, 5923–5941. [Google Scholar] [CrossRef]
- Rilda, Y.; Pernando, D.; Arief, S.; Syukri, S.; Refinel, R.; Agustien, A.; Pardi, H. The use of the low-temperature sol–gel method for ZnO-TiO2 nanorods synthesis: Structural analysis, morphology and photodegradation properties of methyl orange dye with benzoquinone scavenger. J. Iran. Chem. Soc. 2022, 19, 2023–2030. [Google Scholar] [CrossRef]
- Pant, H.R.; Pant, B.; Sharma, R.K.; Amarjargal, A.; Kim, H.J.; Park, C.H.; Tijing, L.D.; Kim, C.S. Antibacterial and photocatalytic properties of Ag/TiO2/ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.L.; Zhang, W.Z.; Wang, L.L.; Chen, X.; Gao, Y. Microwave-assisted synthesis of nanocomposite Ag/ZnO–TiO2 and photocatalytic degradation Rhodamine B with different modes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 134–141. [Google Scholar] [CrossRef]
- Shalaby, A.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Dimitriev, Y.; Stoyanova, A.; Hitkova, H.; Ivanova, N.; Sredkova, M. Sol-gel synthesis and properties of nanocomposites in the Ag/TiO2/ZnO system. J. Optoelectron. Adv. Mater. 2015, 17, 248–256. [Google Scholar]
- Nien, Y.H.; Hu, G.M.; Rangasamy, M.; Yong, Z.R.; Chou, J.C.; Lai, C.H.; Kuo, P.Y.; Chang, J.X.; Lin, Y.C. Investigation on Photoanode Modified with TiO2–ZnO–Ag Nanofibers in Dye-Sensitized Solar Cell Under Different Intensities of Illuminations. IEEE Trans. Electron Devices 2020, 67, 4983–4989. [Google Scholar] [CrossRef]
- Song, J.; Sun, G.; Yu, J.Y.; Si, Y.; Ding, B. Construction of ternary Ag@ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification. Ceram. Int. 2020, 46, 468–475. [Google Scholar] [CrossRef]
- Elsellami, L.; Djeridi, W. Charge transfer modulation (e−/h+) between TiO2, ZnO and Ag for a superior photocatalytic performance. J. Mater. Sci. Res. 2022, 37, 897–908. [Google Scholar] [CrossRef]
- Kerli, S.; Kavgaci, M.; Soguksu, A.K.; Avar, B. Photocatalytic Degradation of Methylene Blue, Rhodamine-B, and Malachite Green by Ag @ ZnO/TiO2. Braz. J. Phys. 2022, 52, 22. [Google Scholar] [CrossRef]
- Chen, W.J.; Hsu, K.C.; Fang, T.H.; Chen, T.H.; Li, M.H. Characteristics and heterostructure of metal-doped TiO2/ZnO nanocatalysts. Curr. Appl. Phys. 2022, 38, 1–6. [Google Scholar] [CrossRef]
- Štular, D.; Savio, E.; Simončič, B.; Sobak, M.; Jerman, I.; Poljanšek, I.; Ferri, A.; Tomšič, B. Multifunctional antibacterial and ultraviolet protective cotton cellulose developed by in situ biosynthesis of silver nanoparticles into a polysiloxane matrix mediated by sumac leaf extract. Appl. Surf. Sci. 2021, 563, 150361. [Google Scholar] [CrossRef]
- Filipič, J.; Glažar, D.; Jerebic, S.; Kenda, D.; Modic, A.; Roskar, B.; Vrhovski, I.; Štular, D.; Golja, B.; Smolej, S.; et al. Tailoring of Antibacterial and UV-protective Cotton Fabric by an in situ Synthesis of Silver Particles in the Presence of a Sol-gel Matrix and Sumac Leaf Extract. Tekstilec 2020, 63, 4–13. [Google Scholar] [CrossRef]
- ASTM E2149-01; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions (Withdrawn 2010). ASTM International: West Conshohocken, PA, USA, 2001; pp. 1–4.
- EN 13758-1:2002; Textiles. Solar UV Protective Properties Method of Test for Apparel Fabrics. European Committee for Standardization: Brussels, Belgium, 2002; 16p.
- AS/NZS 4399:2017; Australian/New Zealand Standard: Sun Protective Clothing–Evaluation and Classification. Council of Standards New Zealand: Sydney, Australia, 2017.
- Berger-Schunn, A. Practical Color Measurements: A Primer for the Beginner, a Reminder for the Experts; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Socrates, G. Infrared Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons Ltd.: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Zhao, H.B.; Kwak, J.H.; Zhang, Z.C.; Brown, H.M.; Arey, B.W.; Holladay, J.E. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr. Polym. 2007, 68, 235–241. [Google Scholar] [CrossRef]
- Johari, N.D.; Rosli, Z.M.; Juoi, J.M.; Yazid, S.A. Comparison on the TiO2 crystalline phases deposited via dip and spin coating using green sol-gel route. Jmr&T 2019, 8, 2350–2358. [Google Scholar] [CrossRef]
- Verbič, A.; Brenčič, K.; Dolenec, M.; Primc, G.; Recek, N.; Šala, M.; Gorjanc, M. Designing UV-protective and hydrophilic or hydrophobic cotton fabrics through in-situ ZnO synthesis using biodegradable waste extracts. Appl. Surf. Sci. 2022, 599, 153931. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Karkare, M.M. The Direct transition and not Indirect transition, is more favourable for Band Gap calculation of Anatase TiO2 nanoparticles. Int. J. Sci. Eng. 2015, 6, 48–53. [Google Scholar]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]











| Sample | Concentration (mg/kg) | ||
|---|---|---|---|
| TiO2 | ZnO | Ag | |
| CO/TiO2 | 14,680 | 0 | 0 |
| CO/ZnO | 0 | 16,180 | 0 |
| CO/TiO2 + ZnO | 7670 | 9460 | 0 |
| CO/Ag | 0 | 0 | 340 |
| CO/TiO2/Ag | 4170 | 0 | 450 |
| CO/ZnO/Ag | 0 | 12,430 | 710 |
| CO/TiO2 + ZnO/Ag | 1180 | 1320 | 640 |
| Sample | Number of Washings | T (UVA) (%) | T (UVB) (%) | T (UVR) (%) | Mean UPF Value | UVR Protection Category * |
|---|---|---|---|---|---|---|
| CO | 0 | 37.8 | 35.5 | 37.2 | 2.7 | NR |
| 25 | 35.3 | 27.4 | 33.44 | 3.4 | NR | |
| CO/TiO2 | 0 | 13.1 | 4.0 | 11.0 | 20.6 | G |
| 25 | 28.4 | 17.3 | 25.8 | 5.2 | NR | |
| CO/ZnO | 0 | 6.4 | 1.5 | 5.3 | 55.7 | E |
| 25 | 26.9 | 19.6 | 25.2 | 4.7 | NR | |
| CO/TiO2 + ZnO | 0 | 13.0 | 6.0 | 11.4 | 15.9 | G |
| 25 | 26.7 | 16.6 | 24.3 | 5.4 | NR | |
| CO/Ag | 0 | 3.3 | 1.9 | 2.9 | 47.9 | E |
| 25 | 6.4 | 2.8 | 4.9 | 33.2 | VG | |
| CO/TiO2/Ag | 0 | 1.9 | 0.9 | 1.7 | 92.1 | E |
| 25 | 2.7 | 1.5 | 2.4 | 61.0 | E | |
| CO/ZnO/Ag | 0 | 0.5 | 0.4 | 0.5 | 229.0 | E |
| 25 | 0.7 | 0.6 | 0.7 | 164.9 | E | |
| CO/TiO2 + ZnO/Ag | 0 | 1.5 | 0.8 | 1.3 | 105.4 | E |
| 25 | 2.6 | 1.7 | 2.4 | 55.3 | E |
| Sample | SE | ||
|---|---|---|---|
| CO/Ag * | 66.4 | 1.1 | |
| CO/TiO2 | 17.9 | ||
| CO/TiO2/Ag | 89.4 | ||
| CO/ZnO | 53.1 | 1.9 | |
| CO/ZnO/Ag | 226.3 | ||
| CO/TiO2 + ZnO | 13.2 | 1.3 | |
| CO/TiO2 + ZnO/Ag | 102.7 |
| Sample | SE | ||
|---|---|---|---|
| CO/Ag * | 31.8 | 1.7 | |
| CO/TiO2 | 1.8 | ||
| CO/TiO2/Ag | 57.9 | ||
| CO/ZnO | 1.3 | 4.9 | |
| CO/ZnO/Ag | 161.5 | ||
| CO/TiO2 + ZnO | 2.0 | 1.5 | |
| CO/TiO2 + ZnO/Ag | 51.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanuša, M.; Kumer, B.; Petrovčič, E.; Štular, D.; Zorc, M.; Jerman, I.; Gorjanc, M.; Tomšič, B.; Simončič, B. Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs. Nanomaterials 2022, 12, 3140. https://doi.org/10.3390/nano12183140
Ivanuša M, Kumer B, Petrovčič E, Štular D, Zorc M, Jerman I, Gorjanc M, Tomšič B, Simončič B. Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs. Nanomaterials. 2022; 12(18):3140. https://doi.org/10.3390/nano12183140
Chicago/Turabian StyleIvanuša, Monika, Blažka Kumer, Elizabeta Petrovčič, Danaja Štular, Matija Zorc, Ivan Jerman, Marija Gorjanc, Brigita Tomšič, and Barbara Simončič. 2022. "Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs" Nanomaterials 12, no. 18: 3140. https://doi.org/10.3390/nano12183140
APA StyleIvanuša, M., Kumer, B., Petrovčič, E., Štular, D., Zorc, M., Jerman, I., Gorjanc, M., Tomšič, B., & Simončič, B. (2022). Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs. Nanomaterials, 12(18), 3140. https://doi.org/10.3390/nano12183140