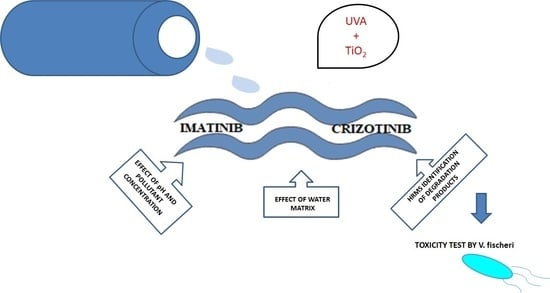
Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Photocatalytic Study
2.3. HPLC Analysis
2.4. Identification of Degradation Products
2.5. Toxicity Experiments
3. Results
3.1. Influence of Process Parameters: pH and Initial Concentration of Drug
3.2. Effect of Water Constituents on IMT and CRZ Degradation
3.2.1. Nitrate Ions
3.2.2. Bicarbonate Ions
3.2.3. Phosphate Ions
3.2.4. Chloride Ions
3.2.5. Humic Acids (HAs) as Dissolved Organic Matter
3.3. Scavenger Study
3.4. Structure Elucidation of Degradation Products




3.5. Toxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Calzaa, P.; Medana, C.; Sarro, M.; Rosato, V.; Aigotti, R.; Baiocchi, C.; Mineroa, C. Photocatalytic degradation of selected anticancer drugs and identification of their transformation products in water by liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2014, 1362, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Secrétan, P.H.; Karoui, M.; Sadou-Yaye, H.; Levi, Y.; Tortolano, L.; Solgadi, A.; Yagoubi, N.; Do, B. Imatinib: Major photocatalytic degradation pathways in aqueous media and the relative toxicity of its transformation products. Sci. Total Environ. 2019, 655, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Tolić, K.; Runje, M.; Gazivoda Kraljević, T.; Mutavdžić Pavlović, D. Identification of crizotinib major degradation products obtained under stress conditions by RP-UHPLC-HRMS. Croat. Chem. Acta 2021, 94, 17–24. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Christophoridis, C.; Fountoulakis, I.; Antonopoulou, M.; Vlastos, D.; Bais, A.; Fytianos, K. Photolytic and photocatalytic degradation of antineoplastic drug irinotecan. Kinetic study, identification of transformation products and toxicity evaluation. Chem. Eng. J. 2021, 405, 126866. [Google Scholar] [CrossRef]
- Havlíkova, L.; Satínský, D.; Solich, P. Aspects of decontamination of ivermectin and praziquantel from environmental waters using advanced oxidation technology. Chemosphere 2016, 144, 21–28. [Google Scholar] [CrossRef]
- Nassour, C.; Barton, S.J.; Nabhani-Gebara, S.; Saab, Z.; Barker, J. Occurrence of anticancer drugs in the aquatic environment: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 1339–1347. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- de Santiago-Martín, A.; Meffe, R.; Teijón, G.; Martínez Hernández, V.; López-Heras, I.; Alonso, C.; Arenas Romasanta, M.; de Bustamante, I. Pharmaceuticals and trace metals in the surface water used for crop irrigation: Risk to health or natural attenuation? Sci. Total Environ. 2020, 705, 135825. [Google Scholar] [CrossRef]
- Periša, M.; Babić, S. Farmaceutici u okolišu. Kem. Ind. 2015, 65, 471–482. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, S.; Jiang, W.; Yue, H.; Cui, Z.; Liang, B. Adsorption and photocatalytic degradation behaviors of rhodamine dyes on surface fluorinated TiO2 under visible irradiation. RSC Adv. 2016, 6, 4090–4100. [Google Scholar] [CrossRef]
- Fonseca-Cervantes, O.R.; Pérez-Larios, A.; Romero Arellano, V.H.; Sulbaran-Rangel, B.; Guzmán González, C.A. Effects in band gap for photocatalysis in TiO2 support by adding gold and ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Wender, H.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A., Jr. Photocatalytic treatment of metoprolol with B-doped TiO2: Effect of water matrix, toxicological evaluation and identification of intermediates. Appl. Catal. B 2015, 176, 173–182. [Google Scholar] [CrossRef]
- Babić, S.; Zrnčić, M.; Ljubas, D.; Ćurković, L.; Škorić, I. Photolytic and thin TiO2 film assisted photocatalytic degradation of sulfamethazine in aqueous solution. Environ. Sci. Pollut. Res. 2015, 22, 11372–11386. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.D.; Lee, J.Y.; Kim, C.H.; Lee, H.S.; Koo, S.M.; Lee, Y.J.; Paik, J.-H.; Kim, D.Y.; Kong, S.H. A study on TiO2 surface texturing effect for the enhancement of photocatalytic reaction in a total phosphorous concentration measurement system. Micromachines 2021, 12, 1163. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilized form for the photocatalytic degradation of nitrobenzene sulfonic acids. Appl. Catal. B 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Dijkstra, M.F.J.; Michorius, A.; Buwalda, H.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C.M. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Radjenović, J.; Sirtori, C.; Petrović, M.; Barceló, D.; Malato, S. Solar photocatalytic degradation of persistent pharmaceuticals at pilot-scale: Kinetics and characterization of major intermediate products. Appl. Catal. B 2009, 89, 255–264. [Google Scholar] [CrossRef]
- Sheng, H.; Li, Q.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Photocatalytic degradation of organic pollutants on surface anionized TiO2: Common effect of anions for high hole-availability by water. Appl. Catal. B 2013, 138–139, 212–218. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Rapid degradation of clozapine by heterogeneous photocatalysis. Comparison with direct photolysis, kinetics, identification of transformation products and scavenger study. Sci. Total Environ. 2019, 665, 557–567. [Google Scholar] [CrossRef]
- Schneider, J.T.; Scheres Firak, D.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723. [Google Scholar] [CrossRef]
- Gambín, M.; Pérez-Lucas, G.; Navarro, S. Removal kinetics of four leacher herbicides through solar heterogeneous photocatalysis as influenced by water matrix. Bull. Environ. Contam. Toxicol. B 2021, 106, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Liang, M.; Zheng, H.; Zhu, C.; Wang, Y. Detection of imatinib based on electrochemical sensor constructed using biosynthesized graphene-silver nanocomposites. Front. Chem. 2021, 9, 670074. [Google Scholar] [CrossRef] [PubMed]
- Mišík, M.; Filipic, M.; Nersesyan, A.; Kundi, M.; Isidori, M.; Knasmueller, S. Environmental risk assessment of widely used anticancer drugs (5-fluorouracil, cisplatin, etoposide, imatinib mesylate). Water Res. 2019, 164, 114953. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer 2013, 2, 91–97. [Google Scholar] [PubMed]
- Gouveia, T.I.A.; Alves, A.; Santos, M.S.F. New insights on cytostatic drug risk assessment in aquatic environments based on measured concentrations in surface waters. Environ. Int. 2019, 133, 105236. [Google Scholar] [CrossRef]
- Mihçiokur, H. Environmental risk assessment of commonly used anti-cancer drugs. Cumhuriyet Sci. J. 2021, 42, 310–320. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.-Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef]
- Grčić, I.; Marčec, J.; Radetić, L.; Radovan, A.M.; Melnjak, I.; Jajčinović, I.; Brnardić, I. Ammonia and methane oxidation on TiO2 supported on glass fiber mesh under artificial solar irradiation. Environ. Sci. Pollut. Res. 2020, 28, 18354–18367. [Google Scholar] [CrossRef]
- Biošić, M.; Škorić, I.; Beganović, J.; Babić, S. Nitrofurantoin hydrolytic degradation in the environment. Chemosphere 2017, 186, 660–668. [Google Scholar] [CrossRef]
- Tolić Čop, K.; Mutavdžić Pavlović, D.; Duić, K.; Pranjić, M.; Fereža, I.; Jajčinović, I.; Brnardić, I.; Špada, V. Sorption potential of different forms of TiO2 for the removal of two anticancer drugs from water. Appl. Sci. 2022, 12, 4113. [Google Scholar] [CrossRef]
- Malinowski, S.; Presečki, I.; Jajčinović, I.; Brnardić, I.; Mandić, V.; Grčić, I. Intensification of dyhidroxybenzenes degradation over immobilized TiO2 based photocatalysts under simulated solar light. Appl. Sci. 2020, 10, 7571. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals: Test No. 111: Hydrolysis as a 722 Function of pH 1–15; OECD: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 173–1746. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Gharaghani, M.A. Photocatalytic degradation of ciprofloxacin antibiotic by TiO2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun. 2019, 207, 56–72. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef]
- Qamar, M.; Muneer, M.; Bahnemann, D. Heterogeneous photocatalysed degradation of two selected pesticide derivatives, triclopyr and daminozid in aqueous suspensions of titanium dioxide. J. Environ. Manag. 2006, 80, 99–106. [Google Scholar] [CrossRef]
- Mioduszewska, K.; Dołżonek, J.; Wyrzykowski, D.; Kubik, Ł.; Wiczling, P.; Sikorska, C.; Toński, M.; Kaczyński, Z.; Stepnowski, P.; Białk-Bielińska, A. Overview of experimental and computational methods for the determination of the pKa values of 5-fluorouracil, cyclophosphamide, ifosfamide, imatinib. TrAC Trends Anal. Chem. 2017, 97, 283–296. [Google Scholar] [CrossRef]
- Bandla, J.; Ganapaty, S. Stability indicating UPLC method development and validation for the determination of crizotinib in pharmaceutical dosage forms. Int. J. Sci. Prog. Res. 2018, 9, 1493–1498. [Google Scholar]
- Parmar, N.; Srivastava, J.K. Degradation of pharmaceutical antibiotic (ciprofloxacin) by photocatalysis process using sol-gel based titanium dioxide nanoparticles. Int. J. Chem. React. Eng. 2021, 19, 929–938. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaart, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Xu, J.; Hao, Z.; Guo, C.; Zhang, Y.; Meng, W. Photodegradation of sulfapyridine under simulated sunlight irradiation: Kinetics, mechanism and toxicity evolvement. Chemosphere 2014, 99, 186–191. [Google Scholar] [CrossRef]
- Dugandžić, A.M.; Tomašević, A.V.; Radišić, M.M.; Šekuljica, N.Ž.; Mijin, D.Ž.; Petrović, S.D. Effect of inorganic ions, photosensitisers and scavengers on the photocatalytic degradation of nicosulfuron. J. Photochem. Photobiol. 2017, 336, 146–155. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Peng, W.; Luo, Y.; He, D. Effects of inorganic ions on the photocatalytic degradation of carbamazepine. J. Water Reuse Desalin. 2019, 9, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Ismail, L.; Ferronato, C.; Fine, L.; Jaber, F.; Chovelon, J.-M. Effect of water constituents on the degradation of sulfaclozine in the three systems: UV/TiO2, UV/K2S2O8, and UV/TiO2/K2S2O8. Environ. Sci. Pollut. Res. 2018, 25, 2651–2663. [Google Scholar] [CrossRef]
- Dabić, D.; Babić, S.; Škorić, I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere 2019, 230, 268–277. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Shi, H.; Miao, D.; Yang, Y.; Qian, L. Photolysis of astorvastatin in aquatic environment: Influencing factors, products, and pathways. Chemosphere 2018, 212, 467–475. [Google Scholar] [CrossRef]
- Lam, M.W.; Tantuco, K.; Mabury, S. PhotoFate: A new approach in accounting for the contribution of indirect photolysis of pesticides and pharmaceuticals in surface waters. Environ. Sci. Technol. 2003, 37, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Meng, C.; Zeng, C.; Ji, Y.; Yang, X.; Gao, S. The effect of nitrate, bicarbonate and natural organic matter on the degradation of sunscreen agent p-aminobenzoic acid by simulated solar irradiation. Sci. Total Environ. 2011, 409, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface modification of TiO2 by phosphate: effect on photocatalytic activity and mechanism implication. J. Phys. Chem. 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Liao, H.; Reitberger, T. Generation of free OHaq radicals by black light illumination of Degussa (Evonik) P25 TiO2 aqueous suspensions. Catalysts 2013, 3, 418–443. [Google Scholar] [CrossRef]
- van Doorslaer, X.; Dewulf, J.; de Maerschalk, J.; van Langenhove, H.; Demeestere, K. Heterogeneous photocatalysis of moxifloxacin in hospital effluent: Effect of selected matrix constituents. Chem. Eng. J. 2015, 261, 9–16. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of water matrix on photocatalytic degradation and general kinetic modelling. Appl. Catal. B 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Chu, W.; Gao, N.; Li, C.; Cui, J. Photochemical degradation of typical halogenated herbicide 2,4-D in drinking water with UV/H2O2/micro-aeration. Sci. China Ser. B Chem. 2009, 52, 2351. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shead, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LR under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Márquez, G.; Tena, M.; Álvarez, P.M.; Beltrán, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Wilczewska, P.; Ona, A.E.N.; Bielicka-Giełdońa, A.; Malankowska, A.; Tabaka, K.; Ryl, J.; Pniewski, F.; Siedlecka, E.M. Application of BiOClnBrm photocatalyst to cytostatic drugs removal from water; mechanism and toxicity assessment. Sep. Purif. Technol. 2021, 254, 117601. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Ofiarska, A.; Borzyszkowska, A.F.; Białk-Bielińska, A.; Stepnowski, P.; Pieczyńska, A. Cytostatic drug removal using electrochemical oxidation with BDD electrode: Degradation pathway and toxicity. Water Res. 2018, 144, 235–245. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpä, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Crane, M.; Watts, C.; Boucard, T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci. Total Environ. 2006, 367, 23–41. [Google Scholar] [CrossRef]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef]
- Zrnčević, S. Farmaceutici i metode obrade otpadne vode iz farmaceutske industrije, pregledni članak. Hrvatske Vode 2016, 24, 119–136. [Google Scholar]
- Mano, B.; Jesus, F.; Gonçalves, F.J.M.; Ventura, S.P.M.; Pereira, J.L. Applicability of heuristic rules defining structure–ecotoxicity relationships of ionic liquids: An integrative assessment using species sensitivity distributions (SSD). Green. Chem. 2020, 22, 6176–6186. [Google Scholar] [CrossRef]











| No. | Parameters | First Order Degradation Rate, k (min−1) | ||
|---|---|---|---|---|
| pH | γ (Pollutant), mg/L | IMT | CRZ | |
| 1 | 5 | 5 | 0.0168 | 0.1397 |
| 2 | 7 | 5 | 0.0154 | 0.1234 |
| 3 | 9 | 5 | 0.0148 | 0.0962 |
| 4 | 5 | 10 | 0.0137 | 0.0718 |
| 5 | 7 | 10 | 0.0115 | 0.0690 |
| 6 | 9 | 10 | 0.0111 | 0.0421 |
| 7 | 5 | 15 | 0.0148 | 0.1041 |
| 8 | 7 | 15 | 0.0135 | 0.0815 |
| 9 | 9 | 15 | 0.0113 | 0.0533 |
| PhAc | Model Equation | Statistical Data | Influencing Model Factor (Based on p-Value) | Influencing Parameters | ||
|---|---|---|---|---|---|---|
| R2 | R2adj | p | ||||
| IMT | k = 0.012 − 1.33·10−3A − 1.217·10−3B − 3.5·10−4AB + 3·10−4A2 + 2.35·10−3B2 | 0.9813 | 0.9517 | 0.0081 | A, B, B2 | pH, γ(IMT) |
| CRZ | k = 0.065 − 0.021A − 0.020B − 1.825·10−3 − 6.767·10−3A2 + 0.039B2 | 0.9840 | 0.9572 | 0.0068 | A, B, B2 | pH, γ(CRZ) |
| IMT | CRZ | ||||||
|---|---|---|---|---|---|---|---|
| Water Constituent | Concentration, mg/L | k, min−1 | R2 | t1/2, min | k, min−1 | R2 | t1/2, min |
| No addition | 0 | 0.0168 | 0.9912 | 41.26 | 0.1380 | 0.9767 | 5.02 |
| Nitrate | 5 25 50 | 0.0115 0.0106 0.0091 | 0.9810 0.9768 0.9924 | 60.27 65.39 76.17 | 0.1361 0.0813 0.0523 | 0.9806 0.9848 0.9902 | 5.09 8.53 13.25 |
| Bicarbonate | 50 100 250 | 0.0167 0.0178 0.0128 | 0.9892 0.9827 0.9838 | 41.51 38.94 54.15 | 0.0157 0.0188 0.0809 | 0.9406 0.9408 0.9338 | 44.15 36.87 8.57 |
| Chloride | 10 100 1000 | 0.0134 0.0113 0.0053 | 0.9853 0.9473 0.9590 | 51.73 61.34 130.78 | 0.0584 0.0307 0.0496 | 0.9635 0.9654 0.9582 | 11.87 22.58 13.97 |
| Phosphate | 10 50 100 | 0.011 0.0126 0.0124 | 0.9753 0.9796 0.9617 | 63.01 55.01 55.90 | 0.0221 0.0255 0.0257 | 0.9346 0.9326 0.9330 | 31.36 27.18 26.97 |
| Humic acids | 5 10 20 | 0.0113 0.0066 0.0091 | 0.9927 0.9910 0.9728 | 61.34 105.02 76.17 | 0.0332 0.0099 0.0052 | 0.9509 0.9647 0.9484 | 20.88 70.01 133.30 |
| IMT | CRZ | |||||
|---|---|---|---|---|---|---|
| Experiment | k, min−1 | R2 | t1/2, min | k, min−1 | R2 | t1/2, min |
| PhAc + water matrix in Milli-Q | 0.0131 | 0.9493 | 52.91 | 0.0281 | 0.9886 | 24.67 |
| PhAc + wastewater | 0.0094 | 0.9947 | 73.74 | 0.0265 0.0027 | 0.9863 0.9019 | 26.15 256.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolić Čop, K.; Mutavdžić Pavlović, D.; Gazivoda Kraljević, T. Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs. Nanomaterials 2022, 12, 3532. https://doi.org/10.3390/nano12193532
Tolić Čop K, Mutavdžić Pavlović D, Gazivoda Kraljević T. Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs. Nanomaterials. 2022; 12(19):3532. https://doi.org/10.3390/nano12193532
Chicago/Turabian StyleTolić Čop, Kristina, Dragana Mutavdžić Pavlović, and Tatjana Gazivoda Kraljević. 2022. "Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs" Nanomaterials 12, no. 19: 3532. https://doi.org/10.3390/nano12193532
APA StyleTolić Čop, K., Mutavdžić Pavlović, D., & Gazivoda Kraljević, T. (2022). Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs. Nanomaterials, 12(19), 3532. https://doi.org/10.3390/nano12193532