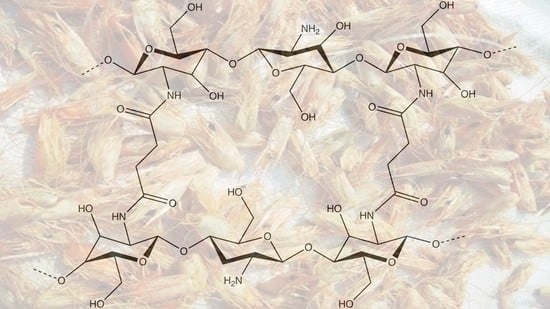
Crosslinked Chitosan Binder for Sustainable Aqueous Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crosslinked Binder
2.2. Electrode Materials Synthesis and Electrode Preparation
2.2.1. Na2Ti2(PO4)3 Synthesis
2.2.2. Electrode Preparation
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Binder Characterization
3.2. Electrode Preparation and Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Physicochemical Characterization of NaTi2(PO4)3 (NTP)


Appendix A.2. Electron Microscopy Characterization of NaTi2(PO4)3 (NTP)

Appendix A.3. Solubility Tests

Appendix A.4. Chitosan SEM Images

Appendix A.5. Electrochemical Stability Window of the Electrolytes

Appendix A.6. Electrochemical Tests of AC Electrodes Prepared in Different Conditions

References
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Ruffo, R.; Wessel, C.; Huggins, R.A.; Cui, Y. Electrochemical behavior of LiCoO2 as aqueous lithium-ion battery electrodes. Electrochem. Commun. 2009, 11, 247–249. [Google Scholar] [CrossRef]
- Li, W.; McKinnon, W.R.; Dahn, J.R. Lithium Intercalation from Aqueous Solutions. J. Electrochem. Soc. 1994, 141, 2310–2316. [Google Scholar] [CrossRef]
- Chen, L.; Cao, L.; Ji, X.; Hou, S.; Li, Q.; Chen, L.; Yang, C.; Eidson, N.; Wang, C. Enabling safe aqueous lithium ion open batteries by suppressing oxygen reduction reaction. Nat. Commun. 2020, 11, 2638. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Q.; Wang, S.W.; Wang, E.; Guo, X.; Chou, S.L. High-Abundance and Low-Cost Metal-Based Cathode Materials for Sodium-Ion Batteries: Problems, Progress, and Key Technologies. Adv. Energy Mater. 2009, 9, 1803609. [Google Scholar] [CrossRef]
- Philippot, M.; Alvarez, G.; Ayerbe, E.; Van Mierlo, J.; Messagie, M. Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs. Batteries 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renew. Sustain. Energ. Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Bresser, D.; Bucholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Bigoni, F.; De Giorgio, F.; Soavi, F.; Arbizzani, C. Sodium alginate: A water-processable binder in high-voltage cathode formulations. J. Electrochem. Soc. 2017, 164, A6171–A6177. [Google Scholar] [CrossRef]
- De Giorgio, F.; La Monaca, A.; Dinter, A.; Frankenberger, M.; Pettinger, K.H.; Arbizzani, C. Water-processable Li4Ti5O12 electrodes featuring eco-friendly sodium alginate binder. Electrochim. Acta 2018, 289, 112–119. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, T.; GE, D.; Wood, D.L., III; Li, Z. Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System. iScience 2020, 23, 101081. [Google Scholar] [CrossRef]
- Toigo, C.; Arbizzani, C.; Pettinger, K.-H.; Biso, M. Study on Different Water-Based Binders for Li4Ti5O12 Electrodes. Molecules 2020, 25, 2443. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhong, X.; Yuan, C.; Duan, L.; Zhang, L.; Wang, Z.; Wang, C.; Shi, F. Sodium Alginate Binders for Bivalency Aqueous Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20681–20688. [Google Scholar] [CrossRef]
- Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zhang, L.; Zheng, H. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
- Versaci, D.; Nasi, R.; Zubair, U.; Amici, J.; Sgroi, M.; Dumitrescu, M.A.; Francia, C.; Bodoardo, S.; Penazzi, N. New eco-friendly low-cost binders for Li-ion anodes. J Solid State Electrochem. 2017, 21, 3429–3435. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Moustafa, M.E.; Yang, H. Ecofriendly Method to Dissolve Chitosan in Plain Water. ACS Biomater. Sci. Eng. 2019, 5, 6355–6360. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pajak, A.; Kolodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y. Facile synthesis of NaTi2(PO4)3-carbon composite through solid state method and its application in aqueous sodium ion battery. Mater. Lett. 2018, 231, 183–186. [Google Scholar] [CrossRef]
- Toigo, C. Towards Eco-Friendly Batteries: Concepts for Lithium and Sodium Ion Batteries. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2021. [Google Scholar]
- Wrobel, N.; Schinkinger, M.; Mirsky, V.M. A Novel Ultraviolet Assay for Testing Side Reactions of Carbodiimides. Anal. Biochem. 2002, 305, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Sedyakina, N.E.; Zakharov, A.N.; Krivoshchepov, A.F.; Pribytkova, A.P.; Bogdanova, Y.A.; Feldman, N.B.; Lutsenko, S.V.; Avramenko, G.V. Effect of carbon chain length of dicarboxylic acids as cross-linking agents on morphology, encapsulation, and release features of protein-loaded chitosan microparticles. Colloid Polym. Sci. 2017, 295, 1915–1924. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Studies on Cross-linking of Succinic Acid with Chitosan/Collagen. Mater. Res. 2013, 16, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Liesivuori, J.; Savolainen, H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, J.; Gai, L.; Ma, X.; Zhou, J. NaTi2 (PO4) 3/C|| LiMn2O4 rechargeable battery operating with Li+/Na+-mixed aqueous electrolyte exhibits superior electrochemical performance. Electrochim. Acta 2017, 255, 220–229. [Google Scholar] [CrossRef]
- Wu, M.; Ni, W.; Hu, J.; Ma, J. NASICON-Structured NaTi2(PO4)3 for Sustainable Energy Storage. Nano-Micro Lett. 2019, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Pleckaityte, G.; Petruleviciene, M.; Staisiunas, L.; Tediashvili, D.; Pilipavicius, J.; Juodkazyte, J.; Vilciauskas, L. Understanding and mitigation of NaTi2(PO4)3 degradation in aqueous Na-ion batteries. J. Mater. Chem. A 2021, 9, 12670–12683. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Pan, N.; Hsieh, Y.-L. High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.; Wu, M.Y.; Ramanujapuram, A.; Benson, J.; Lee, J.T.; Magasinski, A.; Nitta, N.; Huang, C.; Yushin, G. Enhancing Cycle Stability of Lithium Iron Phosphate in Aqueous Electrolytes by Increasing Electrolyte Molarity. Adv. Energy Mater. 2016, 6, 1501805. [Google Scholar] [CrossRef]
- He, P.; Zhang, X.; Wang, Y.-G.; Cheng, L.; Xia, Y.-Y. Lithium-Ion Intercalation Behavior of LiFePO4 in Aqueous and Nonaqueous Electrolyte Solutions. J. Electrochem. Soc. 2008, 155, A144–A150. [Google Scholar] [CrossRef]
- He, P.; Liu, J.-L.; Cui, W.-J.; Luo, J.-Y.; Xia, Y.-Y. Investigation on capacity fading of LiFePO4 in aqueous electrolyte. Electrochim. Acta 2011, 56, 2351–2357. [Google Scholar] [CrossRef]
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S. Recent Progress in Capacity Enhancement of LiFePO4 Cathode for Li-Ion Batteries. J. Electrochem. Energy Conver. Storage 2021, 18, 010801. [Google Scholar] [CrossRef]
- Toigo, C.; Kracalik, M.; Bradt, E.; Pettinger, K.-H.; Arbizzani, C. Rheological Properties of Aqueous Sodium Alginate Slurries for LTO Battery Electrodes. Polymers 2021, 13, 3582. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargnesi, L.; Gigli, F.; Albanelli, N.; Toigo, C.; Arbizzani, C. Crosslinked Chitosan Binder for Sustainable Aqueous Batteries. Nanomaterials 2022, 12, 254. https://doi.org/10.3390/nano12020254
Bargnesi L, Gigli F, Albanelli N, Toigo C, Arbizzani C. Crosslinked Chitosan Binder for Sustainable Aqueous Batteries. Nanomaterials. 2022; 12(2):254. https://doi.org/10.3390/nano12020254
Chicago/Turabian StyleBargnesi, Luca, Federica Gigli, Nicolò Albanelli, Christina Toigo, and Catia Arbizzani. 2022. "Crosslinked Chitosan Binder for Sustainable Aqueous Batteries" Nanomaterials 12, no. 2: 254. https://doi.org/10.3390/nano12020254
APA StyleBargnesi, L., Gigli, F., Albanelli, N., Toigo, C., & Arbizzani, C. (2022). Crosslinked Chitosan Binder for Sustainable Aqueous Batteries. Nanomaterials, 12(2), 254. https://doi.org/10.3390/nano12020254