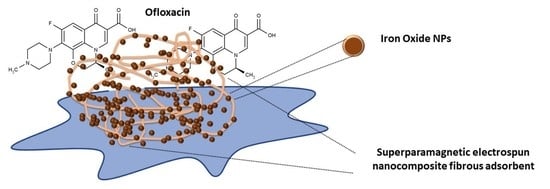
Ofloxacin Removal from Aqueous Media by Means of Magnetoactive Electrospun Fibrous Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Polymer Synthesis
2.3. Membrane Fabrication
2.4. Membrane Characterization
2.5. Adsorption Studies
2.5.1. Adsorption Studies in Synthetic Aqueous Media
2.5.2. Ofloxacin Removal from Urban Wastewater
2.6. Desorption Studies
3. Results
3.1. Polymer Synthesis and Molecular Characterization
3.2. Membrane Fabrication and Characterization
3.2.1. Membrane Fabrication and Morphological Characterization
3.2.2. Determination of the FexOy Nanocrystalline Phase
3.2.3. Magnetic Properties
3.2.4. Mechanical Properties
3.3. Adsorption Studies
3.3.1. Ofloxacin Removal from Synthetic Aqueous Media
3.3.2. Removal of OFL from Urban Wastewater
3.4. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinnaiyan, P.; Thampi, S.G.; Kumar, M.; Mini, K.M. Pharmaceutical products as emerging contaminant in water: Relevance for developing nations and identification of critical compounds for Indian environment. Environ. Monit. Assess. 2018, 190, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Pena, O.I.; Lopez Zavala, M.A.; Ruelas, H.C. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Monaem, E.M.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Abou Alsoaud, M.M.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations. Arab. J. Chem. 2022, 15, 103743–103764. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef] [Green Version]
- Monarca, S.; Feretti, D.; Collivignarelli, C.; Guzzella, L.; Zerbini, I.; Bertanza, G.; Pedrazzani, R. The influence of different disinfectants on mutagenicity and toxicity of urban wastewater. Water Res. 2000, 34, 4261–4269. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment-A review. Environ. Res. 2022, 204, 112298–112313. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment. J. Clean. Prod. 2022, 356, 131826–131861. [Google Scholar] [CrossRef]
- Ullaha, A.; Shahzadab, K.; Wali Khanc, S.; Starov, V. Purification of produced water using oscillatory membrane filtration. Desalination 2020, 491, 114428–114435. [Google Scholar] [CrossRef]
- Kosutic, K.; Dolar, D.; Asperger, D.; Kunst, B. Removal of antibiotics from a model wastewater by RO/NF membranes. Sep. Purif. Technol. 2007, 53, 244–249. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, R.; Asthanaa, A.; Kumar Singh, A.; Jaina, B.; Hasan Susan, A.B. Adsorption of heavy metal ions by various low-cost adsorbents: A review. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608–123630. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef] [PubMed]
- Uddin, Z.; Ahmad, F.; Ullan, T.; Nawab, Y.; Ahmad, S.; Azam, F.; Rasheed, A.; Zafar, M.S. Recent trends in water purifcation using electrospun nanofbrous membranes. Int. J. Environ. Sci. Technol. 2022, 19, 9149–9176. [Google Scholar] [CrossRef]
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, J.; Zhang, X.; Li, W.; Xie, Y.; Sun, S.; Zhao, W.; Zhao, C. Functionalized polyethersulfone nanofibrous membranes with ultra-high adsorption capacity for organic dyes by one-step electrospinning. J. Colloid Interface Sci. 2019, 533, 526–538. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Papaphilippou, P.C.; Vyrides, I.; Mpekris, F.; Stylianopoulos, T.; Papatryfonos, C.A.; Theocharis, C.R.; Krasia-Christoforou, T. Evaluation of novel, cationic electrospun microfibrous membranes as adsorbents in bacteria removal. RSC Adv. 2015, 5, 67617–67629. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total. Environ. 2021, 751, 141673–141691. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. WIREs Nanomed. Nanobiotechnol. 2020, 12, 1611–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 32, 4423–4448. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Gil, A.L.; Yusef, M.; Kenny, J.M.; Peponi, L.P. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef] [PubMed]
- Homocianu, M.; Pascariu, P. Electrospun Polymer-Inorganic Nanostructured Materials and Their Applications. Poly. Rev. 2020, 60, 493–541. [Google Scholar] [CrossRef]
- Patel, S.; Hota, G. Iron oxide nanoparticles immobilized PAN nanofibers: Synthesis and adsorption studies. RSC Adv. 2016, 6, 15402–15414. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Kari, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Philippou, K.; Christou, C.N.; Socoliuc, V.; Vekas, L.; Tanasă, E.; Miclau, M.; Pashalidis, I.; Krasia-Christoforou, T. Superparamagnetic polyvinylpyrrolidone/chitosan/Fe3O4 electrospun nanofibers as effective U(VI) adsorbents. J. Appl. Polym. Sci. 2021, 138, 50212–50226. [Google Scholar] [CrossRef]
- Panteli, S.; Savva, I.; Efstathiou, M.; Vekas, L.; Marinica, O.M.; Krasia-Christoforou, T.; Pashalidis, I. β-Ketoester-Functionalized Magnetoactive Electrospun Polymer Fibers as Eu (III) Adsorbents. SN Appl. Sci. 2019, 1, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Li, X.; Li, Y.; Li, Y.; Sun, B.; Zhang, N.; Chao, S.; Wang, C. Functionalized magnetic iron oxide/polyacrylonitrile composite electrospun fibers as effective chromium (VI) adsorbents for water purification. J. Colloid Interface Sci. 2017, 505, 1018–1030. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Rivas-Rojas, P.; Huck-Iriart, C.; Larrañaga, A.; Fernández-Cirelli, A.; Cerveny, S.; Goyanes, S. Enhancing arsenic adsorption via excellent dispersion of iron oxide nanoparticles inside poly(vinyl alcohol) nanofibers. J. Environ. Chem. Eng. 2021, 9, 104664–104674. [Google Scholar] [CrossRef]
- Savva, I.; Marinica, O.; Papatryfonos, C.A.; Vekas, L.; Krasia-Christoforou, T. Evaluation of electrospun polymer–Fe3O4 nanocomposite mats in malachite green adsorption. RSC Adv. 2015, 5, 16484–16496. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Zhong, L.; Cheng, X. Removal of tetracycline from aqueous solution by a Fe3O4 incorporated PAN electrospun nanofiber mat. J. Environ. Sci. 2015, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mukhortova, Y.R.; Pryadko, A.S.; Chernozem, R.V.; Pariy, I.O.; Akoulina, E.A.; Demianova, I.V.; Zharkova, I.I.; Ivanov, Y.F.; Wagner, D.V.; Bonartsev, A.P.; et al. Fabrication and characterization of a magnetic biocomposite of magnetite nanoparticles and reduced graphene oxide for biomedical applications. Nano-Struct. Nano-Objects 2022, 29, 100843. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P. Advances of graphene oxide based nanocomposite materials in the treatment of wastewater containing heavy metal ions and dyes. Curr. Opin. Green Sustain. Chem. 2022, 5, 100306–100317. [Google Scholar] [CrossRef]
- Mura, S.; Jiang, Y.; Vassalini, I.; Gianoncelli, A.; Alessandri, I.; Granozzi, G.; Calvillo, L.; Senes, N.; Enzo, S.; Innocenzi, P.; et al. Graphene Oxide/Iron Oxide Nanocomposites for Water Remediation. ACS Appl. Nano Mater. 2018, 1, 6724–6732. [Google Scholar] [CrossRef]
- Mong Thy, L.T.; Hoai Thuong, N.; Hoang Tu, T.; Minh Nam, H.; Huu Hieu, N.; Thanh Phong, M. Synthesis of magnetic iron oxide/graphene oxide nanocomposites for removal of cadmium ions from water. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025006–025013. [Google Scholar]
- Su, H.; Ye, Z.; Hmid, N. High-performance iron oxide-graphene oxide nanocomposite adsorbents for arsenic removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 161–172. [Google Scholar] [CrossRef]
- Mong Thya, L.T.; Mai Cuong, P.; Hoang Tua, T.; Minh Nam, H.; Huu Hieua, N.; Thanh Phong, M. Fabrication of Magnetic Iron Oxide/Graphene Oxide Nanocomposites for Removal of Lead Ions from water. Chem. Eng. Trans. 2020, 78, 277–282. [Google Scholar]
- Samie, B.; Toosi, A. Adsorption of malachite green on silica gel: Effects of NaCl, pH and 2-propanol. J. Hazard. Mater. 2010, 184, 739–745. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Yu, J.; Li, S.; Wang, J.; Wang, C.; Chu, F. Integration of lignin and acrylic monomers towards grafted copolymers by free radical polymerization. Int. J. Biol. Macromol. 2014, 67, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Philippou, K.; Krasia-Christoforou, T.; Pashalidis, I. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohydr. Polym. 2019, 219, 298–305. [Google Scholar] [CrossRef]
- Safarik, I.; Pospiskova, K.; Baldikova, E.; Savva, I.; Vekas, L.; Marinica, O.; Tanasa, E.; Krasia-Christoforou, T. Fabrication and Bioapplications of Magnetically Modified Chitosan-based Electrospun Nanofibers. Electrospinning 2018, 2, 29–39. [Google Scholar] [CrossRef]
- Huang, S.J.; Ke, J.H.; Chen, G.J.; Wang, L.F. One-pot synthesis of PDMAEMA-bound iron oxide nanoparticles for magnetofection. J. Mater. Chem. B. 2013, 1, 5916–5924. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Savva, I.; Efstathiou, M.; Krasia-Christoforou, T.; Pashalidis, I. Adsorptive removal of U(VI) and Th(IV) from aqueous solutions using polymer-based electrospun PEO/PLLA fibrous membranes. J. Radioanal. Nucl. Chem. 2013, 298, 1991–1997. [Google Scholar] [CrossRef]
- Starodubtsev, S.G.; Saenko, E.V.; Dokukin, M.E.; Aksenov, V.L.; Klechkovskaya, V.V.; Zanaveskina, I.S.; Khokholov, A.R. Formation of magnetite nanoparticles in poly(acrylamide) gels. J. Phys. Cond. Matter 2005, 17, 1471. [Google Scholar] [CrossRef]
- Gong, T.; Yang, D.; Hu, J.; Yang, W.; Wang, C.; Lu, J.Q. Preparation of monodispersed hybrid nanospheres with high magnetite content from uniform Fe3O4 clusters. Colloids Surf. A Physicochem. Eng. Aspects 2009, 339, 232–239. [Google Scholar] [CrossRef]
- Wan, S.; Zheng, Y.; Liu, Y.; Yan, H.; Liu, K. Fe3O4 nanoparticles coated with homopolymers of glycerol mono(meth)acrylate and their bloc copolymers. J. Mater. Chem. 2005, 15, 3424–3430. [Google Scholar] [CrossRef]
- Papaphilippou, P.C.; Pourgouris, A.; Marinica, O.; Taculescu, A.; Athanasopoulos, G.I.; Vekas, L.; Krasia-Christoforou, T. Fabrication and characterization of superparamagnetic and thermoresponsive hydrogels based on oleic-acid-coated Fe3O4 nanoparticles, hexa(ethylene glycol) methyl ether methacrylate and 2-(acetoacetoxy)ethyl methacrylate. J. Magn. Magn. Mater. 2011, 323, 557–563. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Kantorovich, S.S.; Reznikov, E.N.; Holm, C.; Pshenichnikov, A.F.; Lebedev, A.V.; Chremos, A.; Camp, P.J. Magnetic properties of polydisperse ferrofluids: A critical comparison between experiment, theory, and computer simulation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007, 75, 061405–061417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolmacheva, V.V.; Apyari, V.V.; Ibragimova, B.N.; Kochuk, E.V.; Dmitrienko, S.G.; Zolotov, Y.A. A Polymeric Magnetic Adsorbent Based on Fe3O4 Nanoparticles and Hypercrosslinked Polystyrene for the Preconcentration of Tetracycline Antibiotics. J. Anal. Chem. 2015, 70, 1313–1321. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Y.; Li, Y.; Song, Q. Preparation of Fe3O4/PVP magnetic nanofibers via in situ method with electrospinning. J. Phys. Conf. Ser. 2020, 1549, 03287–03295. [Google Scholar] [CrossRef]
- Sepulveda-Guzman, S.; Lara, L.; Perez-Camacho, O.; Rodrıguez-Fernandez, O.; Olivas, A.; Escudero, R. Synthesis and characterization of an iron oxide poly(styreneco-carboxybutylmaleimide) ferrimagnetic composite. Polymer 2007, 48, 720–727. [Google Scholar] [CrossRef]
- Zhu, C.; Lang, Y.; Liu, B.; Zhao, H. Ofloxacin Adsorption on Chitosan/Biochar Composite: Kinetics, Isotherms, and Effects of Solution Chemistry. Polycycl. Aromat. Compd. 2019, 39, 287–297. [Google Scholar] [CrossRef]
- Vojnovic, B.; Cetina, M.; Franjkovic, P.; Sutlovic, A. Influence of Initial pH Value on the Adsorption of Reactive Black 5 Dye on Powdered Activated Carbon: Kinetics, Mechanisms, and Thermodynamics. Molecules 2022, 27, 1349. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of Sulfonamide Antibiotics to Multiwalled Carbon Nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Van Wieren, E.M.; Seymour, M.D.; Peterson, J.W. Interaction of the fluoroquinolone antibiotic, ofloxacin, with titanium oxide nanoparticles in water: Adsorption and breakdown. Sci. Total Environ. 2012, 441, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, L.B.; Zhao, Q.B.; Frear, C.; Zheng, Y.M. Synthesis of Fe3O4/Polyacrylonitrile Composite Electrospun Nanofiber Mat for Effective Adsorption of Tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 14573–14583. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G. Interaction of Tetracycline with Aluminum and Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Streat, M.; Newton, N.L.R. Hydrous ferric oxide as an adsorbent in water treatment: Part 1. Preparation and physical characterization. Process Saf. Environ. Prot. 2008, 86, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Lu, Z.; Wang, Y.; Wang, Y.; Wan, S. Enhanced Removal of Heavy Metals from Water by Hydrous Ferric Oxide-Modified Biochar. ACS Omega 2020, 5, 28702–28711. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Atassi, Y. Adsorption of methylene blue onto electrospun nanofbrous membranes of polylactic acid and polyacrylonitrile coated with chloride doped polyaniline. Sci. Rep. 2020, 10, 13412–13432. [Google Scholar] [CrossRef] [PubMed]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaphilippou, P.C.; Marinica, O.M.; Tanasă, E.; Mpekris, F.; Stylianopoulos, T.; Socoliuc, V.; Krasia-Christoforou, T. Ofloxacin Removal from Aqueous Media by Means of Magnetoactive Electrospun Fibrous Adsorbents. Nanomaterials 2022, 12, 3648. https://doi.org/10.3390/nano12203648
Papaphilippou PC, Marinica OM, Tanasă E, Mpekris F, Stylianopoulos T, Socoliuc V, Krasia-Christoforou T. Ofloxacin Removal from Aqueous Media by Means of Magnetoactive Electrospun Fibrous Adsorbents. Nanomaterials. 2022; 12(20):3648. https://doi.org/10.3390/nano12203648
Chicago/Turabian StylePapaphilippou, Petri Ch., Oana Maria Marinica, Eugenia Tanasă, Fotios Mpekris, Triantafyllos Stylianopoulos, Vlad Socoliuc, and Theodora Krasia-Christoforou. 2022. "Ofloxacin Removal from Aqueous Media by Means of Magnetoactive Electrospun Fibrous Adsorbents" Nanomaterials 12, no. 20: 3648. https://doi.org/10.3390/nano12203648
APA StylePapaphilippou, P. C., Marinica, O. M., Tanasă, E., Mpekris, F., Stylianopoulos, T., Socoliuc, V., & Krasia-Christoforou, T. (2022). Ofloxacin Removal from Aqueous Media by Means of Magnetoactive Electrospun Fibrous Adsorbents. Nanomaterials, 12(20), 3648. https://doi.org/10.3390/nano12203648