Facile Preparation of MCM-41/Ag2O Nanomaterials with High Iodide-Removal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
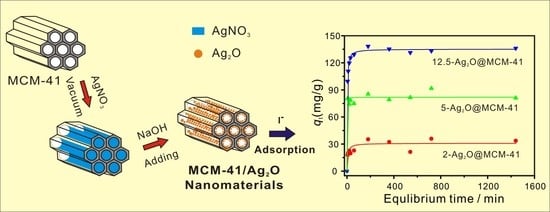
2.2. Preparation of MCM-41/Ag2O Nanomaterials
2.3. Characterization Methods
2.4. Iodide Adsorption Tests
3. Results and Discussion
3.1. Characterization of Samples
3.2. Iodide Adsorption Performance of Samples
3.3. Iodide Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 Nanobelts Heterostructure with Enhanced Ultraviolet and Visible Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, N.; Zhang, H.; Tian, W.; Zhang, T.; Wu, P.; Jiang, W. Visible-light-driven photocatalysis-assisted adsorption of azo dyes using Ag2O. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124105. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Liu, Y.; Fang, P.; Dai, Y. Enhanced adsorption and photocatalytic degradation of high-concentration methylene blue on Ag2O-modified TiO2-based nanosheet. Chem. Eng. J. 2013, 221, 283–291. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Gui, Y.; Ran, L.; Chen, X. Adsorption properties of Ag2O–MoSe2 towards SF6 decomposed products. Vacuum 2021, 189, 110248. [Google Scholar] [CrossRef]
- Gao, R.; Lu, Y.; Xiao, S.; Li, J. Facile Fabrication of Nanofibrillated Chitin/Ag2O Heterostructured Aerogels with High Iodine Capture Efficiency. Sci. Rep. 2017, 7, 4303. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O–Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Satayeva, A.; Yagofarova, A.; Tauanov, Z.; Meiramkulova, K.; Farrando-Perez, J.; Bear, J.C. Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials 2020, 10, 1156. [Google Scholar] [CrossRef]
- Zia, M.R.; Raza, M.A.; Park, S.H.; Irfan, N.; Ahmed, R.; Park, J.E.; Jeon, J.; Mushtaq, S. Removal of Radioactive Iodine Using Silver/Iron Oxide Composite Nanoadsorbents. Nanomaterials 2021, 11, 588. [Google Scholar] [CrossRef]
- Mnasri, N.; Charnay, C.; de Ménorval, L.-C.; Moussaoui, Y.; Elaloui, E.; Zajac, J. Silver nanoparticle-containing submicron-in-size mesoporous silica-based systems for iodine entrapment and immobilization from gas phase. Microporous Mesoporous Mater. 2014, 196, 305–313. [Google Scholar] [CrossRef]
- Yu, W.; Xu, H.; Roden, E.E.; Wan, Q. Efficient adsorption of iodide from water by chrysotile bundles with wedge-shaped nanopores. Appl. Clay Sci. 2019, 183, 105331. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, C.; Creeley, D.; Ho, Y.-F.; Li, H.-P.; Grandbois, R.; Schwehr, K.A.; Kaplan, D.I.; Yeager, C.M.; Wellman, D.; et al. Iodine-129 and Iodine-127 Speciation in Groundwater at the Hanford Site, U.S.: Iodate Incorporation into Calcite. Environ. Sci. Technol. 2013, 47, 9635–9642. [Google Scholar] [CrossRef]
- An, H.; Kweon, S.; Park, S.; Lee, J.; Min, H.K.; Park, M.B. Immobilization of Radioiodine via an Interzeolite Transformation to Iodosodalite. Nanomaterials 2020, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Choung, S.; Um, W.; Kim, M.; Kim, M.G. Uptake Mechanism for Iodine Species to Black Carbon. Environ. Sci. Technol. 2013, 47, 10349–10355. [Google Scholar] [CrossRef] [PubMed]
- Bo, A.; Sarina, S.; Zheng, Z.; Yang, D.; Liu, H.; Zhu, H. Removal of radioactive iodine from water using Ag2O grafted titanate nanolamina as efficient adsorbent. J. Hazard. Mater. 2013, 246, 199–205. [Google Scholar] [CrossRef]
- Yu, W.; Dong, Q.; Yu, W.; Qin, Z.; Nie, X.; Wan, Q.; Chen, X. Preparation of Halloysite/Ag2O Nanomaterials and Their Performance for Iodide Adsorption. Minerals 2022, 12, 304. [Google Scholar] [CrossRef]
- Yu, W.; Wan, Q.; Tan, D.; Yang, S.; Qin, Z.; Nie, X. Removal of iodide from water using halloysite/Ag2O composites as efficient adsorbent. Appl. Clay Sci. 2021, 213, 106241. [Google Scholar] [CrossRef]
- Yang, D.; Sarina, S.; Zhu, H.; Liu, H.; Zheng, Z.; Xie, M.; Smith, S.V.; Komarneni, S. Capture of radioactive cesium and iodide ions from water by using titanate nanofibers and nanotubes. Angew. Chem. 2011, 123, 10782–10786. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.F.; Kuang, Q.; Huang, R.B.; Xie, Z.X.; Zheng, L.S. Shape-dependent antibacterial activities of Ag2O polyhedral particles. Langmuir 2010, 26, 2774–2778. [Google Scholar] [CrossRef]
- Kresge, C.; Leonowicz, M.; Roth, W.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Mano, J.F.; Romão, L.P.C.; Paranhos, C.M. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater. 2020, 291, 109698. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Sohouli, E. Synthesis of the decorated carbon nano onions with aminated MCM-41/Fe3O4 NPs: Morphology and electrochemical sensing performance for methotrexate analysis. Microporous Mesoporous Mater. 2022, 331, 111658. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, Z.; Fang, J.; Wang, Z.; Zuo, S. MCM-41 supported nano-sized CuO-CeO2 for catalytic combustion of chlorobenzene. J. Rare Earths 2020, 38, 933–940. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, G.D.; Meynen, V.; Mertens, M.; Bilba, N.; Cool, P.; Vansant, E.F. ZnO nanoparticles supported on mesoporous MCM-41 and SBA-15: A comparative physicochemical and photocatalytic study. J. Mater. Sci. 2010, 45, 5786–5794. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Zhang, D.; Zhang, W.; Chen, S.; Li, M.; Liang, P. Nanoconfinement of Ag nanoparticles inside mesoporous channels of MCM-41 molecule sieve as a regenerable and H2O resistance sorbent for Hg0 removal in natural gas. Chem. Eng. J. 2019, 361, 139–147. [Google Scholar] [CrossRef]
- Yu, W.; Xu, H.; Tan, D.; Fang, Y.; Roden, E.E.; Wan, Q. Adsorption of iodate on nanosized tubular halloysite. Appl. Clay Sci. 2020, 184, 105407. [Google Scholar] [CrossRef]
- Ikari, M.; Matsui, Y.; Suzuki, Y.; Matsushita, T.; Shirasaki, N. Removal of iodide from water by chlorination and subsequent adsorption on powdered activated carbon. Water Res. 2015, 68, 227–237. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D.; Cao, Y.; Zhang, T.; Mao, Y.; Wang, W.; Wang, Z.; Kawi, S. Catalytic steam reforming of in-situ tar from rice husk over MCM-41 supported LaNiO3 to produce hydrogen rich syngas. Renew. Energy 2020, 161, 408–418. [Google Scholar] [CrossRef]
- Yu, W.; Yuan, P.; Liu, D.; Deng, L.; Yuan, W.; Tao, B.; Cheng, H.; Chen, F. Facile preparation of hierarchically porous diatomite/MFI-type zeolite composites and their performance of benzene adsorption: The effects of NaOH etching pretreatment. J. Hazard. Mater. 2015, 285, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, H.; Luo, X.J. Biomineralization Precursor Carrier System Based on Carboxyl-Functionalized Large Pore Mesoporous Silica Nanoparticles. Curr. Med. Sci. 2020, 40, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Yong, N.L.; Ahmad, A.; Mohammad, A. Synthesis and Characterization of Silver Oxide Nanoparticles by a Novel Method. Int. J. Sci. Eng. Res. 2013, 4, 4. [Google Scholar]
- Allard, B.; Torstenfelt, B.; Andersson, K.; Rydberg, J. Possible Retention of Iodine in the Ground. In Scientific Basis for Nuclear Waste Management; Northrup, C.J.M., Ed.; Springer: Boston, MA, USA, 1980; pp. 673–680. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D.I.; Serne, R.J.; Parker, K.E.; Kutnyakov, I.V. Iodide Sorption to Subsurface Sediments and Illitic Minerals. Environ. Sci. Technol. 2000, 34, 399–405. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Tan, D.-Y.; Liu, K.-K.; Yu, H.-G.; Zhong, Y.-H.; Yuan, A.-H.; Yu, W.-B.; He, H.-P. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu (II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater. 2013, 170, 9–19. [Google Scholar] [CrossRef]
- Liang, L.; Li, L. Adsorption behavior of calcined layered double hydroxides towards removal of iodide contaminants. J. Radioanal. Nucl. Chem. 2007, 273, 221–226. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B Environ. 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Schön, G. ESCA studies of Ag, Ag2O and AgO. Acta Chem. Scand. 1973, 27, 2623–2633. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, M.; Liu, G.; Zhang, L.; He, Y.; Xing, X.; Qian, Z.; Zheng, J.; Xu, C. Enhanced Iodide Removal from Water by Nano-Silver Modified Anion Exchanger. Ind. Eng. Chem. Res. 2018, 57, 17401–17408. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Yu, Z.; Gong, L.; Wang, H.; Yu, B.; Zhang, W.; Jiang, J. Transformation of Porous Organic Cages and Covalent Organic Frameworks with Efficient Iodine Vapor Capture Performance. J. Am. Chem. Soc. 2022, 144, 12390–12399. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere 2016, 164, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.S.; Karanfil, T.; Serkiz, S.M. Removal and Sequestration of Iodide Using Silver-Impregnated Activated Carbon. Environ. Sci. Technol. 2002, 36, 784–789. [Google Scholar] [CrossRef]
- Yu, Z.; Warner, J.A.; Dahlgren, R.A.; Casey, W.H. Reactivity of iodide in volcanic soils and noncrystalline soil constituents. Geochim. Cosmochim. Acta 1996, 60, 4945–4956. [Google Scholar] [CrossRef]
- Lefèvre, G.; Walcarius, A.; Ehrhardt, J.J.; Bessière, J. Sorption of Iodide on Cuprite (Cu2O). Langmuir 2000, 16, 4519–4527. [Google Scholar] [CrossRef]






| Samples | WAg (wt.%) 1 | WAg2O (wt.%) 2 | SBET (m2/g) | VTotal (cm3/g) | Vpore<3.8 nm (cm3/g) |
|---|---|---|---|---|---|
| MCM-41 | -- | -- | 951.0 | 0.7568 | 0.4674 |
| 2-Ag2O@MCM-41 | 0.87 | 0.93 | 724.8 | 0.6157 | 0.2102 |
| 5-Ag2O@MCM-41 | 4.59 | 4.93 | 520.6 | 0.4985 | 0.1553 |
| 12.5-Ag2O@MCM-41 | 8.32 | 8.94 | 395.1 | 0.4000 | 0.1040 |
| Ag2O-NPS | -- | -- | 22.7 | 0.0367 | 0.0018 |
| Composites | Supports | qm (mg/g) | WAg2O (wt. %) 1 | qm-Ag2O (mg/g) 2 | Ref. |
|---|---|---|---|---|---|
| 2-Ag2O@MCM-41 | MCM-41 | 31.1 | 0.93 | 3344.1 | This work |
| 5-Ag2O@MCM-41 | MCM-41 | 83.2 | 4.93 | 1687.6 | This work |
| 12.5-Ag2O@MCM-41 | MCM-41 | 134.6 | 8.94 | 1505.6 | This work |
| Ag2O/halloysite | Halloysite | 57.7 | 6.36 | 907.2 | [16] |
| Ag2O/titanate nanolamina | Titanate nanolamina | 431.8 | 43.29 | 997.5 | [15] |
| Ag2O/titanate nanofibers | Titanate nanofibers | 381.0 | Not given | -- | [18] |
| Ag2O@ChNF aerogels | Chitin-based aerogels | ~304.8 | 31.00 | 983.2 | [6] |
| Ag2O-NPS | -- | 743.9 | 100.00 | 743.9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Dong, Q.; Yu, W.; Wan, Q.; Chen, X. Facile Preparation of MCM-41/Ag2O Nanomaterials with High Iodide-Removal Efficiency. Nanomaterials 2022, 12, 3678. https://doi.org/10.3390/nano12203678
Yu W, Dong Q, Yu W, Wan Q, Chen X. Facile Preparation of MCM-41/Ag2O Nanomaterials with High Iodide-Removal Efficiency. Nanomaterials. 2022; 12(20):3678. https://doi.org/10.3390/nano12203678
Chicago/Turabian StyleYu, Wenlin, Qinpeng Dong, Wenbin Yu, Quan Wan, and Xiuli Chen. 2022. "Facile Preparation of MCM-41/Ag2O Nanomaterials with High Iodide-Removal Efficiency" Nanomaterials 12, no. 20: 3678. https://doi.org/10.3390/nano12203678
APA StyleYu, W., Dong, Q., Yu, W., Wan, Q., & Chen, X. (2022). Facile Preparation of MCM-41/Ag2O Nanomaterials with High Iodide-Removal Efficiency. Nanomaterials, 12(20), 3678. https://doi.org/10.3390/nano12203678