1. Introduction
QDs are semiconductor nanocrystals with remarkable optical properties such as bright photoluminescence that can be tuned with respect to the QD size and that shows low photobleaching. Currently, the main fields of application of QDs are optoelectronics, photovoltaics, lighting, and as biosensors [
1]. For example, they are used as photoluminescent emitters in commercial TV screens and displays, and the development of QD-based light-emitting diodes (LEDs) is currently underway [
2]. They are also expected to be used in the near future for photocatalysis purposes and they have been successfully developed, for instance, for polymerization purposes or for biomass valorization [
3,
4]. These uses, combined with inappropriate recycling, would lead to the release of potentially toxic QDs and/or their degradation products into the environment.
There is currently a great effort to develop safer and more sustainable nanomaterials, which would reduce environmental, health and safety (EHS) concerns, all along the life cycle of the product, while preserving its functionality [
5,
6]. Up to now, the most commonly used QDs have been Cd-based (e.g., CdSe, CdTe), but these are gradually being replaced by less toxic and RoHS-compliant InP QDs [
7]. Although less toxic than CdSe [
8], bulk InP is classified as probably carcinogenic by the International Agency for Research on Cancer [
9]. Moreover, when addressing the toxicity of QDs, one should not only consider pristine QDs, but also the transformation products of QDs throughout their life cycle, especially after having been exposed to environmental conditions mimicking the end-of-life of QD-containing products.
The InP QDs presently on the market are core–shell structures in which the core semiconductor (InP) is passivated by a shell composed of another semiconductor material, typically ZnSe and/or ZnS, which protects the shell from quenching, thus enhancing photoluminescence properties [
10,
11,
12]. Doping the InP core with Zn can further enhance the photoluminescence [
13], as well as using a Zn(Se,S) gradient shell rather than a ZnS shell, because ZnSe shows a lower lattice mismatch with InP than Zn, which limits the formation of defect states at the core–shell interface [
10]. Both approaches are considered as means to increase the performance of QDs, but they can also be regarded as safer and sustainable-by-design approaches. Indeed, reducing the lattice mismatch between the core and the shell ensures better protection of the core. Moreover, alloying the InP QD core with Zn reduces the overall content of potentially toxic elements, i.e., In, via substituting it with a less toxic element, i.e., Zn. Finally, their higher photoluminescence makes it possible to decrease the amount of QDs needed to achieve the same photoluminescence level in the final product.
Like Cd-based QDs, InP QDs are susceptible to photodegradation in environmental conditions, which reduces their photoluminescence and leads to the release of toxic chemicals. After photodegradation, we previously showed that QDs composed of an InZnP [
13] or InZnSP [
14] core (In:Zn, 1:1) capped with a Zn(Se,S) shell show increased toxicity as compared to the corresponding pristine QDs, due to the degradation of the QD structure [
15]. When photodegraded, these QDs form large precipitates composed of In–carboxylate and –phosphate, and Zn–phosphate [
15]. They would be better protected if a thicker shell was grown on their core, further enhancing their photostability. However, increasing the thickness of a Zn(Se,S) shell would imply increasing the Se content of the QD, which is not desired because Se is toxic at high concentrations [
16]. Similar as other groups working on InP Qds (for review, see [
10]), we recently reported the synthesis of InZnP QDs having a reduced In content in their core (InZnP, with an In:Zn ratio of 1:2 rather than 1:1 [
15]) and being coated with three different shell types. These shells are either a single Zn(Se,S) gradient shell, or a double-shell where the Zn(Se,S) shell is further coated with a ZnS shell, either thin or thick [
17]. The toxicity of pristine double-shelled InP QDs is lower than that of the corresponding single-shelled ones, but these QDs show substantial toxicity after aging in environmental conditions [
18].
The objective of this study was to analyze whether these double-shell QDs are more resistant to photodegradation than single-shell QDs, which would ensure that their non-toxic, pristine state is maintained for longer times when exposed to environmental conditions. We assessed their photodegradation kinetics and characterized their degradation products after exposure to environmental conditions, in the aqueous phase. Upon irradiation with UV light, these QDs showed several successive stages of physico-chemical transformation. At each of these stages, the secondary products were characterized using scanning transmission electron microscopy and energy-dispersive spectroscopy, as well as X-ray absorption spectroscopy, which provided the speciation of indium, zinc, and selenium in the QD transformation products throughout the aging process.
2. Materials and Methods
2.1. Chemicals
Unless specifically indicated, all chemicals and reagents were purchased from Sigma Aldrich (Saint-Quentin Fallavier, France) and were >99% pure.
2.2. QD Synthesis and Transfer to the Aqueous Phase
QDs were synthesized as described previously [
17]. Briefly, the InZnP core was synthesized as follows. Indium–myristate was prepared by mixing 6.9 mmol of indium acetate, 21.4 mmol of myristic acid, and 15 mL of octadecene (ODE) in a three-neck flask. The mixture was stirred, then degassed under vacuum for 3 h at 120 °C, then cooled at room temperature. The precipitate was washed with dry hexane (~150 mL), then dried under vacuum. Zinc oleate was prepared by mixing 5 mmol of zinc acetate, 10 mmol of oleic acid, and 9.35 mL ODE in a three-neck flask. After stirring and 1 h of degassing under vacuum at 120 °C, the solution was cooled down to room temperature and the flask was backfilled with Ar. This solution was stored in a glovebox until use. To prepare 0.4 mM TOP-Se and 0.4 M TOP-S stock solutions, we dissolved 2 mmol of selenium or 2 mmol of elemental sulfur in 5 mL trioctylphosphine (TOP). The solutions were stirred for 24 h. The core of InZnP nanocrystals was prepared by mixing 0.1 mmol of In–myristate with 0.2 mmol of Zn–stearate (ZnSt
2) and 8.5 mL of ODE in a three-neck flask. The mixture was degassed for 1 h. Then, the flask was backfilled with Ar. The solution was heated to 300 °C in a molten salt bath and when the temperature reached 100 °C, we swiftly injected 0.1 mmol of (TMS)
3P prepared in 1 mL of ODE. The reaction took place for 20 min, then we let the mixture cool down to 220 °C. A volume of 2.5 mL of the 0.4 M Zn–oleate solution was added dropwise, and then we added a mixture composed of 0.444 mL TOP-Se, 0.5 mL ODE, and 1.57 mL TOP-S stock solutions swiftly. This mixture was heated to 300 °C with a heating ramp of 10 °C per min. After 30 min of gradient shell growth, the reaction was stopped by cooling down to room temperature. This resulted in the QD called “gradient shell”, which was further washed three times by precipitation using methanol/chloroform and acetone (1:1,
v/
v), and redispersed in chloroform. They were stored in hexane. For double-shell QDs preparation, the gradient shell QDs were not cooled to room temperature after synthesis, but rather kept at 230 °C. Zinc ethylxanthane (0.1 mmol) was dissolved in 100 µL of DMF and 1 mL of toluene, then mixed with 0.8 mmol of ZnSt
2 dissolved in 3 mL of ODE. At this step, the Zn–ethylxanthane mixture was injected in the ZnSt
2 solution using a syringe pump so that the injection rate could be strictly controlled. The latter was fixed at 8 mL/h. This reaction was maintained for 30 min or 60 min, to grow a thin or a thick ZnS shell, respectively, on the gradient shell QD, leading to the QDs being called “thin shell” and “thick shell”, respectively. Then the reaction was quenched by lowering the temperature to room temperature. These three types of QDs are represented schematically in
Figure 1.
The QDs were synthesized, then transferred to the aqueous phase using ligand exchange with D-penicillamine, as previously described [
17]. For this purpose, a solution of 0.2 M D-penicillamine was prepared in MilliQ water and degassed for 15 min with Ar. We then added 200 µL of Tris (2-carboxyethyl) phosphine hydrochloride (TCEP) to the reaction to avoid disulfide bond formation, and adjusted the pH to 9 with a 25 wt% solution of tetramethylammonium hydroxide (TMAOH) prepared in methanol. The solution was degassed for 15 min with Ar. Then, 0.5 mL of this D-penicillamine solution was added to 1 mL of QD suspension at 3–5 µM, also previously degassed. This mixture was biphasic. It was stirred for 45 min at room temperature, then centrifuged to separate the two phases. The upper phase, which contained the QDs, was sampled and purified using a NAP10 size exclusion column (Sephadex G-25 DNA grade, GFischer Scientific, Illkirch, France). This removed excess ligands and remaining traces of chemical products from the synthesis. The columns were calibrated either with phosphate-buffered saline (1X PBS) buffer or with deionized water; the same were used as eluent and storage mediums for the QDs. They were kept at 4 °C in the dark and under an argon atmosphere, in order to reduce as much exposure as possible to oxygen from the air and to UV light. In these conditions, they remained stable for 1 year when transferred in PBS and for more than 3 years when transferred in ultrapure water, without any change in their optical properties or any sign of precipitation. Their physico-chemical characteristics (primary size, composition, size distribution, nr. of shells, zeta potential, photophysical properties) are summarized in
Table S1 and cryo-transmission electron microscopy (cryo-TEM) images, X-ray diffraction patterns and FT-IR analyses are shown in
Figure 2. As expected, thick shell QDs are larger than gradient shell QDs (
Figure 2A,B), and the diffraction peaks shift to larger angles with increasing ZnS shell thickness (
Figure 2C) due to the higher compressive strain, leading to a lower lattice parameter of the heterostructure. Moreover, the FTIR spectrum shows that, in comparison with free penicillamine (Pen, black), the sulfhydryl signal at around 2550 cm
−1 is absent after coordination to the QD surface (
Figure 2D). The COO
− asymmetric stretching band remains predominant at 1600 cm
−1, while the NH
+ bending signal at 1500 cm
−1 becomes much lower in bound pen. The COO
− symmetric stretch exhibits a slight shift from 1400 to 1410 cm
−1. (N.B.: The bands at around 2350 cm
−1 correspond to CO
2 from the working atmosphere.)
More characterizations of these QDs (elemental composition based on EDX analysis, XPS spectra) are available in our previously published article describing their synthesis with more details [
17].
2.3. Accelerated Weathering in a Climatic Chamber
The QDs were weathered in a Q-SUN Xe-1 Xenon arc test chamber (Q-lab, Bolton, UK) that provides a full spectrum sunlight. An irradiance of 1.4 W/cm² and a temperature of 40 °C were used for these weathering, following the ISO norms 4892-1 (2000) and 4892-2 (2013) and corresponding to the weather conditions of a sunny day at noon in the equator region. QDs were diluted to 1 µM in PBS or MilliQ water (2 mL total volume), and weathered in 10 mm × 10 mm quartz cuvettes that were closed with a screw cap. All QDs were aged for 24 h in these conditions with direct measurement and sample collection at different aging times, for further characterization.
2.4. Characterization of QD Properties
QD size was measured on cryogenic-TEM images, obtained with a FEI (Eindhoven, Netherlands) Polara microscope operating at 300 kV and images were recorded on a Gatan (Elancourt, France) K2 camera. Samples were prepared by depositing 4 µL of diluted QDs onto a 400-mesh copper grid coated with a homemade carbon film. Size distribution was measured as Z-average and polydispersity index (PdI) via dynamic light scattering on a nanoZS zetasizer (Malvern, Palaiseau, France). Their elemental composition was analyzed by energy-dispersive X-ray analysis (EDX) using an Ultra 55+ scanning electron microscope (Zeiss, Marly le Roi, France) equipped with an EDX probe. For this analysis, a concentrated drop of QDs was deposited on a clean silicon substrate and allowed to dry at room temperature. Photoluminescence quantum yield was measured using an integration sphere (Hamamatsu, Massy, France, Quantaurus Absolute PL quantum yield spectrometer) at room temperature, on the QD colloidal solution. The UV-visible absorbance (UV-vis abs) was measured using an 8452A spectrometer (Hewlett Packard, Puteaux, France) and the steady-state photoluminescence (PL) was measured using a Fluorolog FL3-22 spectrometer (Horiba-Jobin Yvon, Palaiseau, France) equipped with a 450 W xenon lamp and a double-grating monochromator for excitation and emission, as well as a photomultiplier tube (R928 Hamamatsu, Massy, France) for detection. Absorption and emission spectra were measured at 0 min, 15 min, 30 min, 45 min, 1 h, 1 h 30, 2 h, 4 h, 6 h, 24 h of aging. Their crystal structure was determined using a Panalytical X’Pert diffractometer equipped with a copper anode (λKα1 = 1.5406 A and λKα2 = 1.5444 A) and including a X’Celerator 1D detector, and FT-IR spectra were obtained using a Bruker (Wissembourg, France) ALPHA E, 200,396 spectrometer.
2.5. Cell Culture and Cytotoxicity Assay
Human skins were obtained from breast surgery of healthy female donors with their informed consent (Centre Hospitalier Universitaire de Grenoble, France), and following the French Public Health Code, article L1245-2 on the use of surgical waste for research purpose. The samples were treated anonymously, and their collection and storage were declared according to the French law, under the CODECOH DC-2008-444 document. Keratinocytes were extracted from these skins using a previously reported procedure [
19] and then grown in Keratinocyte serum-free medium (KSF-M) supplemented with 25 μg/mL bovine pituitary extract, 1.5 ng/mL epidermal growth factor (EGF) and 75 μg/mL primocin. They were maintained at 37 °C and 5% CO
2 in a humidified atmosphere. These cells were used between passage 1 and 3. For cytotoxicity evaluation, cells were seeded in transparent 96-well plates at 25,000 cells per well and exposed 24 h later to QD suspensions at concentrations ranging between 3.125 and 100 nM. Cells exposed to KSF-M were only used as a control, and cells exposed to Triton 1% were used as a positive control. After 24 h of exposure, 50 µL of exposure medium was sampled from each well and transferred to a clean 96-well plate. Lactate dehydrogenase (LDH) release was quantified using In Vitro Toxicology Assay Kit, Lactic Dehydrogenase based (Merck Sigma-Aldrich, St Quentin Fallavier, France) following the manufacturer’s instructions. The cytotoxicity experiment was repeated three times on keratinocytes from three distinct donors (
n = 3). Each of these experiments included five technical replicates per concentration. Statistical analysis was performed using R-studio, first via the Kruskal-Wallis test, then using pairwise comparison via the Wilcoxon rank sum test. A result was considered statistically significant (*) when
p < 0.05.
2.6. Scanning Transmission Electron Microscopy Coupled to Energy-Dispersive X-ray Analysis
Pristine and aged QDs were imaged using scanning transmission electron microscopy (STEM) with a FEI/Tecnai OSIRIS microscope (Eindhoven, The Netherlands) operated at 200 kV. A droplet of QDs was deposited on a copper grid coated with a carbon film, then allowed to dry for 24 h at room temperature. Samples were stored in the dark until imaging. Imaging was performed in the high angle annular dark field (HAADF) mode, and the selected area was analyzed using energy-dispersive X-ray analysis (EDX).
2.7. X-ray Absorption Spectroscopy
After 30 min, 2 h, 6 h and 24 h of aging, significant changes of the QDs’ physico-chemical properties were observed, such as aggregation and sedimentation or color modification. Therefore, aged QDs were collected at these time-points for further analysis by X-ray absorption spectroscopy (XAS). When aggregation was observed macroscopically, samples were centrifuged at 1000× rcf for 2 min and the pellet and supernatant were sampled separately and mixed with 20% glycerol. These samples were placed in the dedicated sample holder and immediately immersed in liquid nitrogen. They were stored in liquid nitrogen until analysis. Zinc speciation was analyzed on the FAME (BM30B) beamline at the European synchrotron radiation facility (ESRF, Grenoble, France) [
20] using the previously reported protocol and settings [
15]. For these analyses, the beamline was operated in a multi-bunch mode (150 to 200 mA). Indium and selenium speciation were similarly analyzed at In K-edge and Se K-edge on the SAMBA beamline at the SOLEIL synchrotron (Optimized Light Source of Intermediate Energy to LURE, Saclay, France) [
21] operating at 450 mA in top-up mode. The SAMBA beamline is equipped with a Si(220) monochromator running in a continuous scan acquisition mode, while FAME beamline is equipped with a 35-element Ge detector (Canberra, Montigny-le-Bretonneux, France). On both FAME and SAMBA beamlines, the samples were analyzed in a liquid He cryostat, i.e., at 20 K. Ten to forty spectra of 3 min each were averaged, depending on the signal. For Se, we observed photoreduction of the samples aged for 24 h due to the beam irradiation, therefore only the five first spectra were averaged for further signal analysis. In all other samples, no radiation damage was observed. Spectra analysis was performed as follows. First, the spectra recorded on each sample were calibrated by setting the absorption edge at 12,658.0 and 27,940.0 eV, thanks to the use of spectra recorded simultaneously on reference Se and In foils. Then, these spectra were normalized using the Demeter software package, version 9.26 [
22]. Finally, shell fitting was used to analyze the spectra from pristine QDs and linear combination fitting (LCF) for aged QDs analysis, using the Demeter/ARTEMIS and ATHENA softwares version 9.26, respectively [
22]. For LCF, we used the following reference spectra: pristine QDs, In acetate and In phosphate (In K-edge), ZnS (bulk and nano), hopeite (Zn
3(PO
4)
2, 4H
2O), Zn phosphate dehydrate (Zn
3(PO
4)
2, 2H
2O) and Zn-reacted hydroxylapatite at pH 6 as previously described [
15], Zn–GSH (1:10 mol:mol, pH 4.5), and Zn–malate as a proxy for Zn–carboxylate (Zn-COOH) (Zn K-edge). Reference spectra used for selenium speciation were red selenium (red Se(0)), grey selenium (grey Se(0)), sodium selenite (Na
2SeO
3), sodium selenate (Na
2SeO
4), selenium dioxide (SeO
2), zinc selenide (ZnSe), selenium disulfide(SeS
2) selenocystine, selenomethionine, and selenourea. Spectra for the aged QDs were analyzed by LCF using pristine, red Se(0) and Na
2SeO
3 reference spectra.
4. Discussion
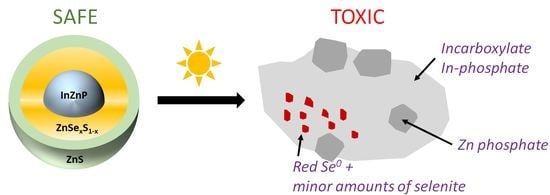
In the present article, we analyze the physico-chemical transformation of three different InP QDs when exposed to artificial weathering under simulated sunlight, which leads to a significant increase in their cytotoxicity.
Our main conclusions are that when exposed to simulated sunlight, QDs lose their absorption and photoluminescence as they degrade. Gradient shell QDs degrade more rapidly than thin shell QDs, themselves degrading more rapidly than thick shell QDs. This behavior confirms the greater photostability, i.e., better resistance to photodegradation in environmental conditions, of double-shell QDs as compared to the single, gradient shell QDs. Since pristine QDs show minor cytotoxicity while aged QDs are highly cytotoxic, avoiding QD degradation is an efficient measure to reduce their hazard. Our results also confirm that thick shell QDs show the highest initial brightness. Therefore, double-shelled QDs with a thick outer shell are good safer-by-design QD candidates as compared to the two other QDs, since their functionality, i.e., photoluminescence, is better and their potential toxicity would be reduced due to their lower photodegradability. During the weathering process, we first observe QDs settling down, while their fluorescence properties remain intact if they are re-dispersed by shaking the suspension. Our hypothesis is that it is certainly due to loss of surface ligands, as previously described for CdSe QDs [
20].
Longer weathering times lead to the progressive chemical degradation of these three QDs, with the formation of secondary products showing distinct optical properties. Aging of CdSe QDs in acidic or alkaline conditions has been shown to induce degradation of the surface coating and release of Cd ions due to the oxidation of Se [
20,
21]. The InP QDs studied here also contain the oxidation-sensitive elements Se and S, therefore some redox reactions can be expected during weathering, and indeed EXAFS analysis proves that oxidation of Se occurs. This would potentially damage QD outer shell layers, i.e., weaken QD core protection, allowing ligands to reach the core, and subsequently lead to its gradual degradation.
Regarding the transformation products, EXAFS analysis shows that In and Zn released from QDs form complexes with phosphates and carboxylates. According to Pearson’s principle (HSAB principle), In
3+ is a hard acid and Zn
2+ is a borderline (intermediate) acid [
31]. Therefore, these two elements preferentially complex with hard bases, including carboxylates and phosphates [
31]. The precipitates observed in the EDX analyses of QDs aged in PBS suggest that Zn precipitates with phosphates (co-localization of Zn, P, and O in the same regions) and that In precipitates with carboxylates, phosphates, and thiols (co-localization of In, C, P, S, and O). A segregation of In and Zn is observed in these precipitates, which can be attributed to their different affinity for the complexing agents. Moreover, the stability constant of hoepite, which is the most frequently reported Zn–phosphate species, is 10
−35 [
22], while the stability constant of In–orthophosphate is 10
−25 [
23]. Such difference would lead to distinct precipitation kinetics, which can explain the observed segregation of In–phosphate and Zn–phosphate precipitates. In comparison, when QDs are aged in water, such segregation of In and Zn precipitation does not occur. It is probable that, in this condition, In and Zn ions form aquo or hydroxide species that co-precipitate simultaneously.
The source of phosphates and carboxylates that complex In and Zn ions when QDs degrade can be multiple. QDs are coated with penicillamine that bear one –COOH and one –SH moiety. Moreover, weathering is performed in aerobic conditions, i.e., in the presence of O2 and CO2 from ambient air, and CO2 can dissolve in the QD suspension and would be another source of carboxylates. Furthermore, QDs are weathered in phosphate buffer saline, which contains 10 mM of phosphate. The contribution of phosphate resulting from the oxidation of indium phosphide is also possible. However, the phosphate concentration in the PBS buffer (~10 mM) is around 50 times higher than the concentration of P in the QDs (~1 µM, around 200 P atoms per 2.7 nm InZnP core). This suggests that PBS is the most probable source of phosphate ligands. Therefore, our interpretation is that phosphates mainly originate from the PBS buffer in which the QDs are aged and carboxylates from penicillamine and/or CO2 originating from ambient air that might dissolve in the QD suspension.
Based on these results, a scenario of weathering in environmental conditions can be proposed (
Figure 7). In pristine QDs, In is present as indium phosphide (InP) in the core, and some indium selenide (In
2Se
3) or a mixed In(P, Se) is present at the core–shell boundary. Furthermore, an amorphous layer of mixed oxides (InPO
3, InPO
4, In
2O
3, In(OH)
3, InO(OH)) can be expected on the surface of the InZnP core according to Virieux and coworkers [
32]. The latter is formed during the high temperature QD synthesis due to the in situ generation of water induced by the ketonization of fatty acids used for complexing the In and Zn precursors [
32]. This layer is not detected in the EXAFS analysis because the contribution of these oxides must be lower than 10%, which is the limit of detection of a species with this technique [
24]. Zn is bound to P in the InZnP QD core, to S and Se in the ZnSe
xS
1−x phases of the gradient shell, and in the ZnS phase of the thin or thick outer shell, in the expected tetrahedral coordination in all phases. Se is only present in the gradient shell and bound exclusively to Zn. Upon exposure to environmental conditions, some dissolution, oxidation, complexation, and precipitation processes occur.
Up to 30 min of weathering, the precipitation of all types of QDs indicates at least partial desorption of the penicillamine surface ligands inducing QD aggregation. Surprisingly, in this short lapse of time already between 30% (thin shell) and 50% (gradient shell) of In is present as In–carboxylate and phosphate, while only around 15% of Zn is transformed to Zn–phosphate and between 0% (thick shell) and 20% (gradient shell) of Se is oxidized. This result suggests that metal ions can leach from the core prior to the complete shell dissolution. Unfortunately, up to now, most QD stability studies focus on the leaching behavior of the core elements (e.g., Cd
2+ in the case of CdSe/ZnS QDs) due to their higher toxicity, neglecting the evolution of the shell constituents. Nonetheless, a similar behavior has been recently reported by Paydary and Larese–Casanova [
33], who studied the dissolution kinetics of aqueous solutions of CdSe/ZnS QDs in the dark. The simultaneous release of Zn
2+ and Cd was attributed to non-uniform coverage of the CdSe cores by the ZnS shell. Here, considering that after 30 min of aging the thin and thick shell QDs still exhibit similar PL properties in terms of intensity, peak position, and line width as the pristine QDs, indicates that In–carboxylate and In–phosphate generated during this period stems from a small fraction of less well passivated and hence non-emissive QDs in the ensemble.
Only for longer irradiation times starting from 45 min, the decrease in PL intensity is accompanied by a blue shift, which is a clear sign of shrinkage of the emissive core. A completely different situation is observed in the gradient shell QDs, which show a strong decrease in PL intensity and a blue shift already after 15 min of irradiation. After 30 min, already around 50% of the In is transformed to In–carboxylate and In–phosphate, and around 20% of Se
2− is oxidized to Se(0). However, once again only a small amount of Zn (10–15%) is transformed to Zn–phosphate species, which shows that this parameter is not a reliable indicator of the shell dissolution. Several factors can explain the comparably fast degradation kinetics observed for the different QDs in this comparative study [
32]: first, complexing agents (phosphate from the PBS buffer, thiol/amine/carboxylate functions from desorbed penicillamine ligands) can accelerate the dissolution process, and second, the generation of reactive oxygen species (ROS) is favored in the presence of QDs and O
2 under light irradiation [
33]. For longer irradiation times (2 h), also in the case of the thin and thick shell QDs, the QD core rapidly degrades to form In–phosphate and In–carboxylate secondary products. The formation of Se and Zn secondary products occurs later, mainly after 6 h and 24 h, respectively. The thick shell QDs are more resistant to degradation than the thin shell QDs, with some fluorescence remaining after 90 min of aging.
We previously showed that these InP QDs are safe in their pristine form, but show significant cytotoxicity and genotoxicity after aging [
18]. Aged QDs impair the viability, oxidative balance, and DNA integrity in exposed cells, but also the overall cellular metal homeostasis [
18]. The latter can be attributed to the release of In, Zn, and Se ions from degraded QDs. Overall, these results suggest that aged InP QD toxicity is triggered by In(III)-phosphate, In(III)-carboxylate, and Zn(II)-phosphate. Therefore, in order to develop even safer InP QDs, one would need to reduce, as much as possible, the release of such In(III) and Zn(II) forms.
5. Conclusions
We report the progressive degradation of multiple shell InZnP QDs upon exposure to simulated solar light under environmentally relevant conditions, with a gradual loss of their optical properties, i.e., their typical excitonic peak, their bright photoluminescence, and of their crystalline structure. Typically, their PL reaches 10% of its initial value after less than 15 min, 60 min, and more than 90 min for gradient shell, thin shell, and thick shell QD, respectively. The transformation products that form when they degrade are indium–phosphate and indium–carboxylate precipitates, as well as zinc–phosphate precipitates and reduced forms of selenium, both red Se(0) and selenite. QDs with a thick ZnS outer shell show the best resistance to photodegradation. While pristine QDs are not toxic, aged QDs show significant cytotoxicity, with LD50 values of 77 nM for pristine gradient shell QD while all three aged QD samples show a DL50 ranging from 15 to 23 nM. Therefore, QDs with a thick outer shell could be considered as a safer-by-design alternative to the gradient shell and thin shell QDs, because they resist, more than the others, to photodegradation as well as showing better photoluminescence properties, with photoluminescence quantum yields of 24% compared to 18% for gradient shell and thin shell QDs. Still, although more resistant, these QDs tend to degrade when exposed in environmental conditions in the presence of complexing agents such as phosphates from the PBS buffer. Zinc chalcogenide shells are prone to oxidative degradation processes and the use of more robust outer shell materials (e.g., composed of other metal oxides) can be proposed if high environmental stability and lower toxicity of QDs is targeted.